Page 235 - Handbook of Battery Materials
P. 235
204 7 Bromine-Storage Materials
300
Br 2 in aqueous phase / mmol l −1 200
100
0
0 20 40 60 80 100
Zu utilization / %
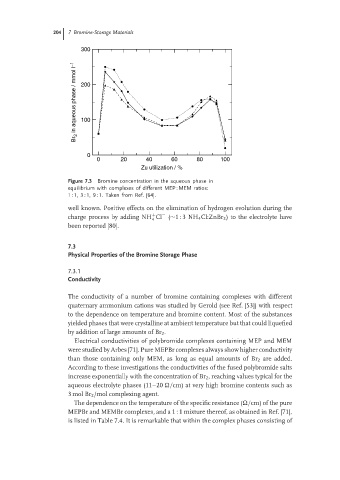
Figure 7.3 Bromine concentration in the aqueous phase in
equilibrium with complexes of different MEP : MEM ratios:
1 : 1, 3 : 1, 9 : 1. Taken from Ref. [64].
well known. Positive effects on the elimination of hydrogen evolution during the
+
charge process by adding NH Cl (∼1:3 NH 4 Cl:ZnBr 2 ) to the electrolyte have
−
4
been reported [80].
7.3
Physical Properties of the Bromine Storage Phase
7.3.1
Conductivity
The conductivity of a number of bromine containing complexes with different
quaternary ammonium cations was studied by Gerold (see Ref. [53]) with respect
to the dependence on temperature and bromine content. Most of the substances
yielded phases that were crystalline at ambient temperature but that could liquefied
by addition of large amounts of Br 2 .
Electrical conductivities of polybromide complexes containing MEP and MEM
were studied by Arbes [71]. Pure MEPBr complexes always show higher conductivity
than those containing only MEM, as long as equal amounts of Br 2 are added.
According to these investigations the conductivities of the fused polybromide salts
increase exponentially with the concentration of Br 2 , reaching values typical for the
aqueous electrolyte phases (11–20 /cm) at very high bromine contents such as
3 mol Br 2 /mol complexing agent.
The dependence on the temperature of the specific resistance ( /cm) of the pure
MEPBr and MEMBr complexes, and a 1 : 1 mixture thereof, as obtained in Ref. [71],
is listed in Table 7.4. It is remarkable that within the complex phases consisting of