Page 234 - Handbook of Battery Materials
P. 234
7.2 Possibilities for Bromine Storage 203
140
120
Vapor pressure / mm Hg 80
100
60
40
20
0
0 10 20 30 40 50 60
temperature / °C
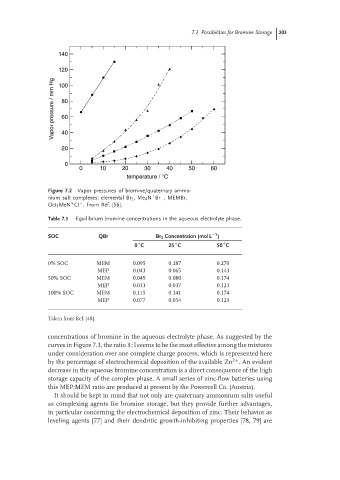
Figure 7.2 Vapor pressures of bromine/quaternary ammo-
nium salt complexes: elemental Br 2 ,Me 4 N Br , MEMBr,
−
+
Oct 3 MeN CI . From Ref. [56].
+
−
Table 7.3 Equilibrium bromine concentrations in the aqueous electrolyte phase.
−1
SOC QBr Br 2 Concentraion (mol L )
◦
◦
◦
0 C 25 C 50 C
0% SOC MEM 0.095 0.187 0.270
MEP 0.043 0.065 0.143
50% SOC MEM 0.049 0.080 0.174
MEP 0.033 0.037 0.123
100% SOC MEM 0.115 0.141 0.174
MEP 0.077 0.054 0.123
Taken from Ref. [48].
concentrations of bromine in the aqueous electrolyte phase. As suggested by the
curves in Figure 7.3, the ratio 3 : l seems to be the most effective among the mixtures
under consideration over one complete charge process, which is represented here
by the percentage of electrochemical deposition of the available Zn .Anevident
2+
decrease in the aqueous bromine concentration is a direct consequence of the high
storage capacity of the complex phase. A small series of zinc-flow batteries using
this MEP:MEM ratio are produced at present by the Powercell Co. (Austria).
It should be kept in mind that not only are quaternary ammonium salts useful
as complexing agents for bromine storage, but they provide further advantages,
in particular concerning the electrochemical deposition of zinc. Their behavior as
leveling agents [77] and their dendritic growth-inhibiting properties [78, 79] are