Page 239 - Handbook of Battery Materials
P. 239
208 7 Bromine-Storage Materials
2
−1
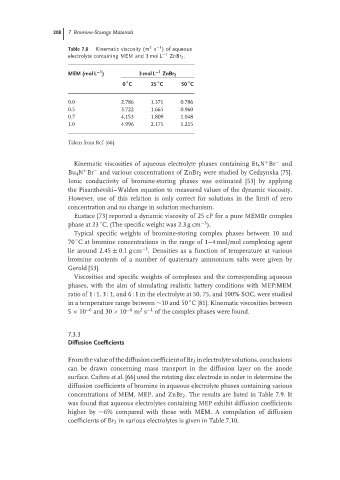
Table 7.8 Kinematic viscosity (m s )of aqueous
electrolyte containing MEM and 3 mol L −1 ZnBr 2 .
−1
MEM (mol L ) 3 mol L −1 ZnBr 2
◦
◦
◦
0 C 25 C 50 C
0.0 2.786 1.371 0.786
0.5 3.722 1.665 0.960
0.7 4.153 1.809 1.048
1.0 4.996 2.175 1.215
Taken from Ref. [66].
−
Kinematic viscosities of aqueous electrolyte phases containing Et 4 N Br and
+
Bu 4 N Br and various concentrations of ZnBr 2 were studied by Cedzynska [75].
−
+
Ionic conductivity of bromine-storing phases was estimated [53] by applying
the Pisarzhevski–Walden equation to measured values of the dynamic viscosity.
However, use of this relation is only correct for solutions in the limit of zero
concentration and no change in solution mechanism.
Eustace [73] reported a dynamic viscosity of 25 cP for a pure MEMBr complex
−3
◦
phase at 23 C. (The specific weight was 2.3 g cm ).
Typical specific weights of bromine-storing complex phases between 10 and
◦
70 C at bromine concentrations in the range of 1–4 mol/mol complexing agent
−3
lie around 2.45 ± 0.1g cm . Densities as a function of temperature at various
bromine contents of a number of quaternary ammonium salts were given by
Gerold [53].
Viscosities and specific weights of complexes and the corresponding aqueous
phases, with the aim of simulating realistic battery conditions with MEP:MEM
ratio of 1 : 1, 3 : 1, and 6 : 1 in the electrolyte at 50, 75, and 100% SOC, were studied
◦
in a temperature range between ∼10 and 50 C [81]. Kinematic viscosities between
2
5 × 10 −6 and 30 × 10 −6 m s −1 of the complex phases were found.
7.3.3
Diffusion Coefficients
From the value of the diffusioncoefficient of Br 2 inelectrolyte solutions,conclusions
can be drawn concerning mass transport in the diffusion layer on the anode
surface. Cathro et al. [66] used the rotating disc electrode in order to determine the
diffusion coefficients of bromine in aqueous electrolyte phases containing various
concentrations of MEM, MEP, and ZnBr 2 . The results are listed in Table 7.9. It
was found that aqueous electrolytes containing MEP exhibit diffusion coefficients
higher by ∼6% compared with those with MEM. A compilation of diffusion
coefficients of Br 2 in various electrolytes is given in Table 7.10.