Page 443 - Handbook of Battery Materials
P. 443
14.8 Examples of Lithium Alloy Systems 415
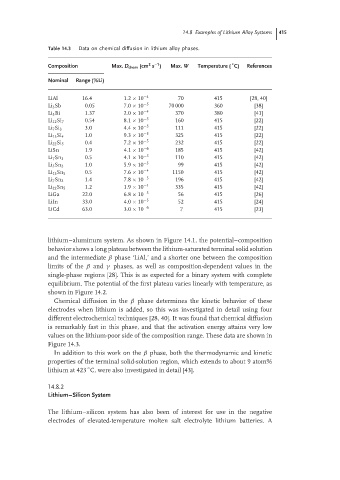
Table 14.3 Data on chemical diffusion in lithium alloy phases.
◦
2
–1
Composition Max. D chem (cm s ) Max. W Temperature ( C) References
Nominal Range (%Li)
LiAl 16.4 1.2 × 10 −4 70 415 [28, 40]
Li 3 Sb 0.05 7.0 × 10 −5 70 000 360 [38]
Li 3 Bi 1.37 2.0 × 10 −4 370 380 [41]
Li 12 Si 7 0.54 8.1 × 10 −5 160 415 [22]
3.0 4.4 × 10 −5 111 415 [22]
Li 7 Si 3
Li 13 Si 4 1.0 9.3 × 10 −5 325 415 [22]
Li 22 Si 5 0.4 7.2 × 10 −5 232 415 [22]
LiSn 1.9 4.1 × 10 −6 185 415 [42]
Li 7 Sn 3 0.5 4.1 × 10 −5 110 415 [42]
Li 5 Sn 2 1.0 5.9 × 10 −5 99 415 [42]
Li 13 Sn 5 0.5 7.6 × 10 −4 1150 415 [42]
Li 7 Sn 2 1.4 7.8 × 10 −5 196 415 [42]
Li 22 Sn 5 1.2 1.9 × 10 −4 335 415 [42]
LiGa 22.0 6.8 × 10 −5 56 415 [26]
LiIn 33.0 4.0 × 10 −5 52 415 [24]
LiCd 63.0 3.0 × 10 −6 7 415 [23]
lithium–aluminum system. As shown in Figure 14.1, the potential–composition
behavior shows a long plateau between the lithium-saturated terminal solid solution
and the intermediate β phase ‘LiAl,’ and a shorter one between the composition
limits of the β and γ phases, as well as composition-dependent values in the
single-phase regions [28]. This is as expected for a binary system with complete
equilibrium. The potential of the first plateau varies linearly with temperature, as
shown in Figure 14.2.
Chemical diffusion in the β phase determines the kinetic behavior of these
electrodes when lithium is added, so this was investigated in detail using four
different electrochemical techniques [28, 40]. It was found that chemical diffusion
is remarkably fast in this phase, and that the activation energy attains very low
values on the lithium-poor side of the composition range. These data are shown in
Figure 14.3.
In addition to this work on the β phase, both the thermodynamic and kinetic
properties of the terminal solid-solution region, which extends to about 9 atom%
◦
lithium at 423 C, were also investigated in detail [43].
14.8.2
Lithium–Silicon System
The lithium–silicon system has also been of interest for use in the negative
electrodes of elevated-temperature molten salt electrolyte lithium batteries. A