Page 447 - Handbook of Battery Materials
P. 447
14.9 Lithium Alloys at Lower Temperatures 419
X (atomic %)
Li
0 50 70 80 85
900 600
LiSn
700 L Li Sn 400
3
7
415 °C
Li Sn Li Sn
2
5
E vs. E Li (mV) 13 Li Sn 2 E vs E Al, ″ LiAi″ (mV)
5
7
Li Sn 5
22
Synthetic Alloys
200 Coulometric Titration
100
0 300
0 1 2 4 5 6
y in Li Sn
y
◦
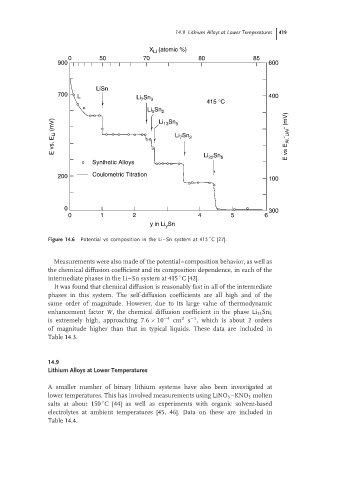
Figure 14.6 Potential vs composition in the Li–Sn system at 415 C [27].
Measurements were also made of the potential–composition behavior, as well as
the chemical diffusion coefficient and its composition dependence, in each of the
intermediate phases in the Li–Sn system at 415 C [42].
◦
It was found that chemical diffusion is reasonably fast in all of the intermediate
phases in this system. The self-diffusion coefficients are all high and of the
same order of magnitude. However, due to its large value of thermodynamic
enhancement factor W, the chemical diffusion coefficient in the phase Li 13 Sn 5
2
−1
is extremely high, approaching 7.6 × 10 −4 cm s , which is about 2 orders
of magnitude higher than that in typical liquids. These data are included in
Table 14.3.
14.9
Lithium Alloys at Lower Temperatures
A smaller number of binary lithium systems have also been investigated at
lower temperatures. This has involved measurements using LiNO 3 –KNO 3 molten
◦
salts at about 150 C [44] as well as experiments with organic solvent-based
electrolytes at ambient temperatures [45, 46]. Data on these are included in
Table 14.4.