Page 530 - Handbook of Battery Materials
P. 530
16.3 SEI Formation on Carbonaceous Electrodes 503
It can be seen that pristine NG7 surface contains mostly (53%) aromatic carbon,
about 20% each of CH and COH groups – only 4.8% of CO groups, and no COOH
groups. The 34% burnt sample consists of mostly CO groups (33%), C–OH groups
(26.6%), and 8.9% of COOH groups.
It was recently found [104] that chemical oxidation of graphite powder by
strong oxidizing agents such as ammonium per-oxysulfate and hot concentrated
nitric acid gave similar results, that is, it suppresses Q IR and enhances Q R to
−1
410–430 mAh g . Following this wet oxidation, carboxyl groups were identified
on the surface of the graphite. Takamura et al. found that heat treatment at 700 C
◦
in the presence of acetylene black improved the performance of the graphite-fiber
anode [105]. HOPG was used as a model electrode for studying separately the
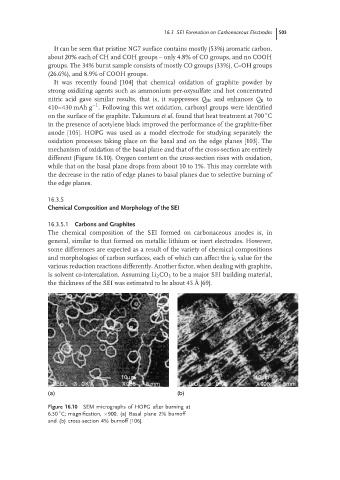
oxidation processes taking place on the basal and on the edge planes [103]. The
mechanism of oxidation of the basal plane and that of the cross-section are entirely
different (Figure 16.10). Oxygen content on the cross-section rises with oxidation,
while that on the basal plane drops from about 10 to 1%. This may correlate with
the decrease in the ratio of edge planes to basal planes due to selective burning of
the edge planes.
16.3.5
Chemical Composition and Morphology of the SEI
16.3.5.1 Carbons and Graphites
The chemical composition of the SEI formed on carbonaceous anodes is, in
general, similar to that formed on metallic lithium or inert electrodes. However,
some differences are expected as a result of the variety of chemical compositions
and morphologies of carbon surfaces, each of which can affect the i 0 value for the
various reduction reactions differently. Another factor, when dealing with graphite,
is solvent co-intercalation. Assuming Li 2 CO 3 to be a major SEI building material,
the thickness of the SEI was estimated to be about 45 ˚ A [69].
10µm 10µm
JEOL 3 . 0KV X908 8 mm JEOL 3 . 0KV X908 1.5mm
(a) (b)
Figure 16.10 SEM micrographs of HOPG after burning at
◦
6.50 C; magnification, ×900. (a) Basal plane 2% burnoff
and (b) cross-section 4% burnoff [106].