Page 525 - Handbook of Battery Materials
P. 525
498 16 The Anode/Electrolyte Interface
In EC - based solution
Blister
Hill
1nm 20 nm
Stable
Passivating
Layer
IN PC Lithium ion
Exfoliation
Solvent molecule
Solvated lithium ion
No
Passivating
Layer
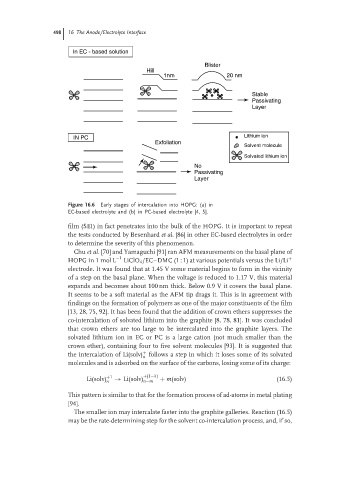
Figure 16.6 Early stages of intercalation into HOPG: (a) in
EC-based electrolyte and (b) in PC-based electrolyte [4, 5].
film (SEI) in fact penetrates into the bulk of the HOPG. It is important to repeat
the tests conducted by Besenhard et al. [86] in other EC-based electrolytes in order
to determine the severity of this phenomenon.
Chu et al. [70] and Yamaguchi [91] ran AFM measurements on the basal plane of
−1
HOPG in 1 mol L LiClO 4 /EC–DMC (1 : 1) at various potentials versus the Li/Li +
electrode. It was found that at 1.45 V some material begins to form in the vicinity
of a step on the basal plane. When the voltage is reduced to 1.17 V, this material
expands and becomes about 100 nm thick. Below 0.9 V it covers the basal plane.
It seems to be a soft material as the AFM tip drags it. This is in agreement with
findings on the formation of polymers as one of the major constituents of the film
[13, 28, 75, 92]. It has been found that the addition of crown ethers suppresses the
co-intercalation of solvated lithium into the graphite [8, 78, 81]. It was concluded
that crown ethers are too large to be intercalated into the graphite layers. The
solvated lithium ion in EC or PC is a large cation (not much smaller than the
crown ether), containing four to five solvent molecules [93]. It is suggested that
the intercalation of Li(solv) follows a step in which it loses some of its solvated
+
n
molecules and is adsorbed on the surface of the carbons, losing some of its charge:
Li(solv) +1 → Li(solv) +(1−∂) + m(solv) (16.5)
n n−m
This pattern is similar to that for the formation process of ad-atoms in metal plating
[94].
The smaller ion may intercalate faster into the graphite galleries. Reaction (16.5)
may be the rate-determining step for the solvent co-intercalation process, and, if so,