Page 521 - Handbook of Battery Materials
P. 521
494 16 The Anode/Electrolyte Interface
In general, lithium-ion batteries are assembled in the discharged state. That
is, the cathode, for example, Li 1 CoO 2 , is fully intercalated by lithium, while the
anode (carbon) is completely empty (not charged by lithium). In the first charge
the anode is polarized in the negative direction (electrons are inserted into the
carbon), and lithium cations leave the cathode, enter the solution, and are inserted
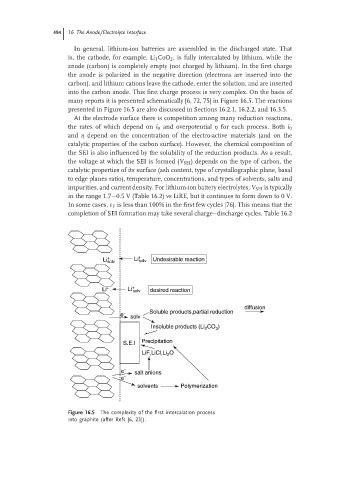
into the carbon anode. This first charge process is very complex. On the basis of
many reports it is presented schematically [6, 72, 75] in Figure 16.5. The reactions
presented in Figure 16.5 are also discussed in Sections 16.2.1, 16.2.2, and 16.3.5.
At the electrode surface there is competition among many reduction reactions,
the rates of which depend on i 0 and overpotential η for each process. Both i 0
and η depend on the concentration of the electro-active materials (and on the
catalytic properties of the carbon surface). However, the chemical composition of
the SEI is also influenced by the solubility of the reduction products. As a result,
the voltage at which the SEI is formed (V SEI ) depends on the type of carbon, the
catalytic properties of its surface (ash content, type of crystallographic plane, basal
to edge planes ratio), temperature, concentrations, and types of solvents, salts and
impurities, and current density. For lithium-ion battery electrolytes, V SEI is typically
in the range 1.7−0.5 V (Table 16.2) vs LiRE, but it continues to form down to 0 V.
In some cases, ε F is less than 100% in the first few cycles [76]. This means that the
completion of SEI formation may take several charge–discharge cycles. Table 16.2
Li + solv Li + solv Undesirable reaction
Li + Li + solv desired reaction
diffusion
e − solv Soluble products,partial reduction
Insoluble products (Li 2 CO 3 )
S.E.I Precipitation
O
LiF,LiCl,Li 2
e − salt anions
e −
solvents Polymerization
Figure 16.5 The complexity of the first intercalation process
into graphite (after Refs [6, 23]).