Page 518 - Handbook of Battery Materials
P. 518
16.3 SEI Formation on Carbonaceous Electrodes 491
species, particle shape and size, pore-size distribution, BET surface area, and
pore-opening are of critical importance in the use of carbons as anode material.
These properties have a major influence on Q IR , reversible capacity Q R ,and therate
capability and safety of the battery. The surface chemical composition depends on
the raw materials (carbon precursors), the production process, and the history of
the carbon. Surface groups containing H, O, S, N, P, halogens, and other elements
have been identified on carbon blacks [64, 65]. There is also ash on the surface
of carbon and this typically contains Ca, Si, Fe, Al, and V. Ash and acidic oxides
enhance the adsorption of the more polar compounds and electrolytes [64].
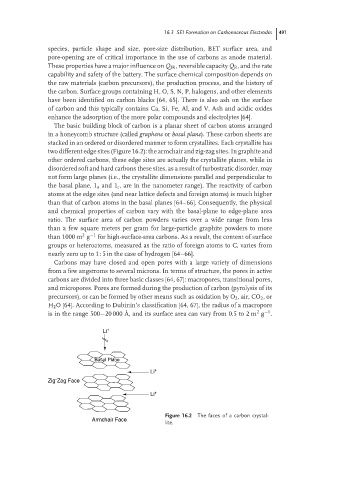
The basic building block of carbon is a planar sheet of carbon atoms arranged
in a honeycomb structure (called graphene or basal plane). These carbon sheets are
stacked in an ordered or disordered manner to form crystallites. Each crystallite has
two different edge sites (Figure 16.2): the armchair and zig-zag sites. In graphite and
other ordered carbons, these edge sites are actually the crystallite planes, while in
disordered soft and hard carbons these sites, as a result of turbostratic disorder, may
not form large planes (i.e., the crystallite dimensions parallel and perpendicular to
the basal plane, 1 a and 1 c , are in the nanometer range). The reactivity of carbon
atoms at the edge sites (and near lattice defects and foreign atoms) is much higher
than that of carbon atoms in the basal planes [64–66]. Consequently, the physical
and chemical properties of carbon vary with the basal-plane to edge-plane area
ratio. The surface area of carbon powders varies over a wide range from less
than a few square meters per gram for large-particle graphite powders to more
2
than 1000 m g −1 for high-surface-area carbons. As a result, the content of surface
groups or heteroatoms, measured as the ratio of foreign atoms to C, varies from
nearly zero up to 1 : 5 in the case of hydrogen [64–66].
Carbons may have closed and open pores with a large variety of dimensions
from a few angstroms to several microns. In terms of structure, the pores in active
carbons are divided into three basic classes [64, 67]: macropores, transitional pores,
and micropores. Pores are formed during the production of carbon (pyrolysis of its
precursors), or can be formed by other means such as oxidation by O 2 , air, CO 2 ,or
H 2 O [64]. According to Dubinin’s classification [64, 67], the radius of a macropore
−1
2
is in the range 500−20 000 ˚ A, and its surface area can vary from 0.5 to 2 m g .
Li +
Basal Plane
Li +
−
Zig Zag Face
Li +
Figure 16.2 The faces of a carbon crystal-
Armchair Face
lite.