Page 582 - Handbook of Battery Materials
P. 582
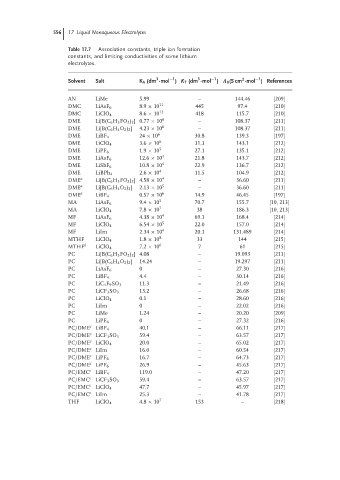
556 17 Liquid Nonaqueous Electrolytes
Table 17.7 Association constants, triple ion formation
constants, and limiting conductivities of some lithium
electrolytes.
3
−1
3
−1
−1
2
Solvent Salt K A (dm ·mol ) K T (dm ·mol ) Λ 0 (S cm ·mol ) References
AN LiMe 5.99 – 144.46 [209]
DMC LiAsF 6 8.9 × 10 11 445 97.4 [210]
DMC LiClO 4 8.6 × 10 12 418 115.7 [210]
DME Li[B(C 6 H 3 FO 2 ) 2 ]0.77 × 10 6 – 108.37 [211]
DME Li[B(C 6 H 4 O 2 ) 2 ] 4.23 × 10 6 – 108.37 [211]
DME LiBF 4 24 × 10 6 30.8 139.3 [197]
DME LiClO 4 3.6 × 10 6 31.3 143.1 [212]
DME LiPF 6 1.9 × 10 5 27.1 135.1 [212]
DME LiAsF 6 12.6 × 10 4 21.8 143.7 [212]
DME LiSbF 6 10.8 × 10 4 22.9 136.7 [212]
DME LiBPh 4 2.6 × 10 4 11.5 104.9 [212]
DME a Li[B(C 6 H 3 FO 2 ) 2 ]4.58 × 10 4 – 36.60 [211]
DME a Li[B(C 6 H 4 O 2 ) 2 ] 2.13 × 10 5 – 36.60 [211]
DME a LiBF 4 0.57 × 10 6 14.9 46.45 [197]
MA LiAsF 6 9.4 × 10 5 70.7 155.7 [10, 213]
MA LiClO 4 7.8 × 10 7 38 186.3 [10, 213]
MF LiAsF 6 4.38 × 10 4 69.1 168.4 [214]
MF LiClO 4 6.54 × 10 5 22.0 157.0 [214]
MF LiIm 2.34 × 10 4 20.1 131.489 [214]
MTHF LiClO 4 1.8 × 10 8 33 144 [215]
MTHF b LiClO 4 7.2 × 10 6 7 61 [215]
PC Li[B(C 6 H 3 FO 2 ) 2 ] 4.08 – 19.093 [211]
PC Li[B(C 6 H 4 O 2 ) 2 ] 14.24 – 19.297 [211]
PC LiAsF 6 0 – 27.30 [216]
PC LiBF 4 4.4 – 30.14 [216]
PC LiC 4 F 9 SO 3 11.3 – 21.49 [216]
PC LiCF 3 SO 3 13.2 – 26.68 [216]
PC LiClO 4 0.1 – 28.60 [216]
PC LiIm 0 – 22.02 [216]
PC LiMe 1.24 – 20.20 [209]
PC LiPF 6 0 – 27.32 [216]
c
PC/DME LiBF 4 40.1 – 66.11 [217]
c
PC/DME LiCF 3 SO 3 59.4 – 63.57 [217]
c
PC/DME LiClO 4 20.0 – 65.02 [217]
c
PC/DME LiIm 16.0 – 60.54 [217]
c
PC/DME LiPF 6 16.7 – 64.73 [217]
c
PC/DME LiPF 6 26.9 – 45.63 [217]
PC/EMC c LiBF 4 119.0 – 47.20 [217]
PC/EMC c LiCF 3 SO 3 59.4 – 63.57 [217]
PC/EMC c LiClO 4 47.7 – 45.97 [217]
PC/EMC c LiIm 25.3 – 41.78 [217]
THF LiClO 4 4.8 × 10 7 153 – [218]