Page 586 - Handbook of Battery Materials
P. 586
560 17 Liquid Nonaqueous Electrolytes
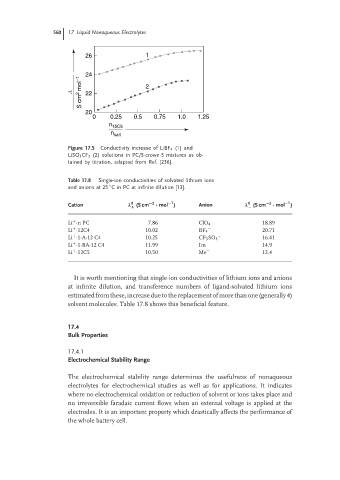
26 1
24
S cm 2 mol −1 2
L 22
20
0 0.25 0.5 0.75 1.0 1.25
n 15C5
n salt
Figure 17.5 Conductivity increase of LiBF 4 (1) and
LiSO 3 CF 3 (2) solutions in PC/5-crown-5 mixtures as ob-
tained by titration, adapted from Ref. [236].
Table 17.8 Single-ion conductivities of solvated lithium ions
◦
and anions at 25 C in PC at infinite dilution [13].
−1
0
−1
0
Cation λ (S cm −2 · mol ) Anion λ (S cm −2 · mol )
+ −
Li ·nPC 7.86 ClO 4 − 18.89
+
+
Li ·12C4 10.02 BF 4 − 20.71
Li ·1-A-12 C4 10.25 CF 3 SO 3 − 16.41
+
+
Li ·1-BA-12 C4 11.99 Im − 14.9
+
Li ·12C5 10.50 Me − 12.4
It is worth mentioning that single-ion conductivities of lithium ions and anions
at infinite dilution, and transference numbers of ligand-solvated lithium ions
estimated from these,increase due to the replacement of more thanone (generally4)
solvent molecules. Table 17.8 shows this beneficial feature.
17.4
Bulk Properties
17.4.1
Electrochemical Stability Range
The electrochemical stability range determines the usefulness of nonaqueous
electrolytes for electrochemical studies as well as for applications. It indicates
where no electrochemical oxidation or reduction of solvent or ions takes place and
no irreversible faradaic current flows when an external voltage is applied at the
electrodes. It is an important property which drastically affects the performance of
the whole battery cell.