Page 591 - Handbook of Battery Materials
P. 591
17.4 Bulk Properties 565
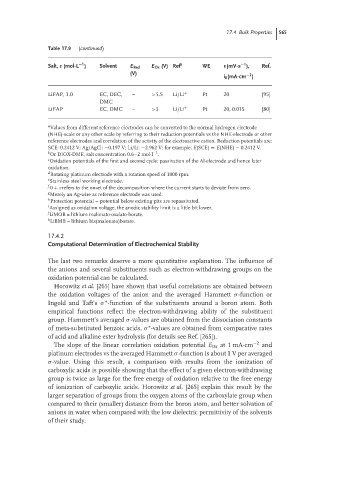
Table 17.9 (continued)
−1
−1
Salt, c (mol·L ) Solvent E Red E Ox (V) Ref a WE v(mV·s ), Ref.
(V)
−2
i 0 (mA·cm )
LiFAP, 1.0 EC, DEC, – >5.5 Li/Li + Pt 20 [95]
DMC
LiFAP EC, DMC – >5 Li/Li + Pt 20, 0.015 [80]
a
Values from different reference electrodes can be converted to the normal hydrogen electrode
(NHE)-scale or any other scale by referring to their reduction potentials vs the NHE-electrode or other
reference electrodes and correlation of the activity of the electroactive cation. Reduction potentials are:
SCE: 0.2412 V; Ag/AgCl: −0.197 V; Li/Li: −2.962 V; for example, E(SCE) = E(NHE) − 0.2412 V.
−1
b Or DIOX-DME, salt concentration 0.6–2 mol·l .
c
Oxidation potentials of the first and second cycle: passivation of the Al-electrode and hence later
oxidation.
d
Rotating platinum electrode with a rotation speed of 1000 rpm.
e Stainless steel working electrode.
f
0 + εrefers to the onset of the decomposition where the current starts to deviate from zero.
g Merely an Ag-wire as reference electrode was used.
h
Protection potential – potential below existing pits are repassivated.
i Assigned as oxidation voltage, the anodic stability limit is a little bit lower.
j
LiMOB = lithium malonato-oxalato-borate.
k LiBMB = lithium bis(malonato)borate.
17.4.2
Computational Determination of Electrochemical Stability
The last two remarks deserve a more quantitative explanation. The influence of
the anions and several substituents such as electron-withdrawing groups on the
oxidation potential can be calculated.
Horowitz et al. [265] have shown that useful correlations are obtained between
the oxidation voltages of the anion and the averaged Hammett σ-function or
Ingold and Taft’s σ -function of the substituents around a boron atom. Both
∗
empirical functions reflect the electron-withdrawing ability of the substituent
group. Hammett’s averaged σ-values are obtained from the dissociation constants
∗
of meta-substituted benzoic acids, σ -values are obtained from comparative rates
of acid and alkaline ester hydrolysis (for details see Ref. [265]).
The slope of the linear correlation oxidation potential E Ox at 1 mA·cm −2 and
platinum electrodes vs the averaged Hammett σ-function is about 1 V per averaged
σ-value. Using this result, a comparison with results from the ionization of
carboxylic acids is possible showing that the effect of a given electron-withdrawing
group is twice as large for the free energy of oxidation relative to the free energy
of ionization of carboxylic acids. Horowitz et al. [265] explain this result by the
larger separation of groups from the oxygen atoms of the carboxylate group when
compared to their (smaller) distance from the boron atom, and better solvation of
anions in water when compared with the low dielectric permittivity of the solvents
of their study.