Page 593 - Handbook of Battery Materials
P. 593
17.4 Bulk Properties 567
• The same effects obtained with lithium phenolate or dilithium-2,2 -biphenyldio-
late based solutions [107].
• Anodic polymer formation from phenols yielding polyphenoxide as already
postulated and proved by Bruno et al. [270].
• Different CVs in the region of the anodic decomposition.
Furthermore, the values of E HOMO give information about the electrochemical
stability of anions with similar structures. The lower E HOMO the more stable is the
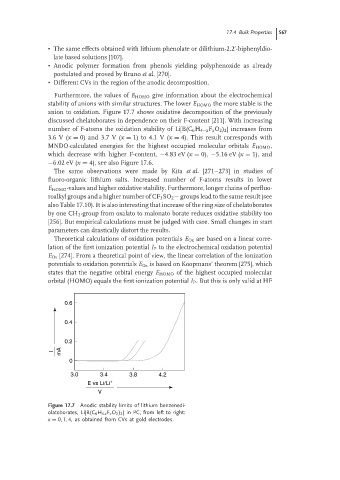
anion to oxidation. Figure 17.7 shows oxidative decomposition of the previously
discussed chelatoborates in dependence on their F-content [211]. With increasing
number of F-atoms the oxidation stability of Li[B(C 6 H 4−x F x O 2 ) 2 ] increases from
3.6 V (x = 0) and 3.7 V (x = 1) to 4.1 V (x = 4). This result corresponds with
MNDO-calculated energies for the highest occupied molecular orbitals E HOMO ,
which decrease with higher F-content, −4.83 eV (x = 0), −5.16 eV (x = 1), and
−6.02 eV (x = 4), see also Figure 17.6.
The same observations were made by Kita et al. [271–273] in studies of
fluoro-organic lithium salts. Increased number of F-atoms results in lower
E HOMO -values and higher oxidative stability. Furthermore, longer chains of perfluo-
roalkyl groups and a higher number of CF 3 SO 2 − groups lead to the same result (see
also Table 17.10). It is also interesting that increase of the ring size of chelatoborates
by one CH 2 -group from oxalato to malonato borate reduces oxidative stability too
[256]. But empirical calculations must be judged with care. Small changes in start
parameters can drastically distort the results.
Theoretical calculations of oxidation potentials E Ox are based on a linear corre-
lation of the first ionization potential I P to the electrochemical oxidation potential
E Ox [274]. From a theoretical point of view, the linear correlation of the ionization
potentials to oxidation potentials E Ox is based on Koopmans’ theorem [275], which
states that the negative orbital energy E HOMO of the highest occupied molecular
orbital (HOMO) equals the first ionization potential I P . But this is only valid at HF
0.6
0.4
0.2
I mA
0
3.0 3.4 3.8 4.2
E vs Li/Li +
V
Figure 17.7 Anodic stability limits of lithium benzenedi-
olatoborates, Li[B(C 6 H 4-x F x O 2 ) 2 ] in PC, from left to right:
x = 0, 1, 4, as obtained from CVs at gold electrodes.