Page 597 - Handbook of Battery Materials
P. 597
17.4 Bulk Properties 571
The passivation of the surface is due to decomposition of the electrolyte and
the following irreversible reaction with the Al surface, as LiPF 6 ,Al 2 O 3 ,and AlF 3
are the main components of the passive layer [286, 287]. X-ray photoelectron spec-
troscopy (XPS) analysis indicates further formation of Al-O and Al-F compounds
on the Al surface, such as Al(OH) 3 , probably from water contamination, Al 2 O 3 , and
AlF 3 [288]. Time-of-flight secondary ion mass spectroscopy (ToF-SIMS) shows a
two-layered structure in which the inner layer consists more of Al-O compounds, for
example, Al 2 O 3 , and the outer layer of Al-F compounds, for example, AlF 3 [289]. For-
mation of AlF 3 arises from reactions of decomposition products of the lithium salt,
mainly HF, with the air-formed and native Al 2 O 3 layer on aluminum [289, 290].
Al 2 O 3 + 6HF → 2AlF 3 + 3H 2 O (17.31)
Al 2 O 3 + 2HF → 2AlOF + H 2 O (17.32)
2AlOF + 2HF → Al 2 OF 4 + H 2 O (17.33)
Al 2 OF 4 + 2HF → 2AlF 3 + H 2 O (17.34)
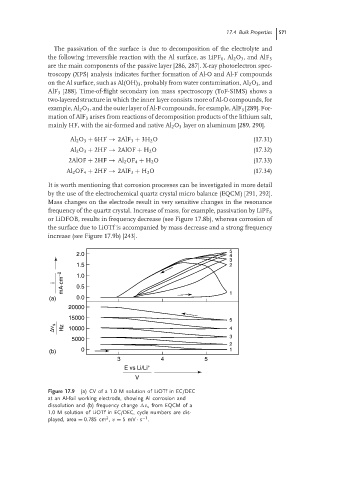
It is worth mentioning that corrosion processes can be investigated in more detail
by the use of the electrochemical quartz crystal micro balance (EQCM) [291, 292].
Mass changes on the electrode result in very sensitive changes in the resonance
frequency of the quartz crystal. Increase of mass, for example, passivation by LiPF 6
or LiDFOB, results in frequency decrease (see Figure 17.8b), whereas corrosion of
the surface due to LiOTf is accompanied by mass decrease and a strong frequency
increase (see Figure 17.9b) [243].
5
2.0 4
3
1.5 2
i mA·cm −2 1.0
0.5
(a) 0.0 1
20000
15000
5
∆ν s Hz 10000 4
3
5000
2
(b) 0 1
3 4 5
E vs Li/Li +
V
Figure 17.9 (a) CV of a 1.0 M solution of LiOTf in EC/DEC
at an Al-foil working electrode, showing Al corrosion and
dissolution and (b) frequency change ν s from EQCM of a
1.0 M solution of LiOTf in EC/DEC, cycle numbers are dis-
2
−1
played, area = 0.785 cm , v = 5mV · s .