Page 592 - Handbook of Battery Materials
P. 592
566 17 Liquid Nonaqueous Electrolytes
A more recent but closely related explanation of the shift of oxidation poten-
tials by electron-withdrawing substituents is based on results of semi-empirical
quantum-mechanical calculations [219, 266–268]. Methods such as MNDO, AM1,
∗
∗
PM3, HF/3-21G , or B3LYP/6-31G are used to yield the energy of the highest
occupied molecular orbital E HOMO of the anion. Correlations of E Ox with E HOMO
are linear for a given set of compounds of similar structure.
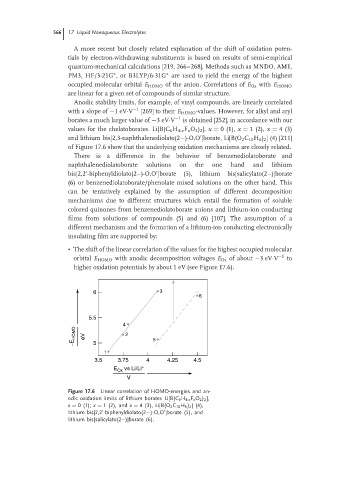
Anodic stability limits, for example, of vinyl compounds, are linearly correlated
with a slope of −1eV·V −1 [269] to their E HOMO -values. However, for alkyl and aryl
borates a much larger value of −3eV·V −1 is obtained [252], in accordance with our
values for the chelatoborates. Li[B(C 6 H 4-x F x O 2 ) 2 ], x = 0(1), x = 1(2), x = 4(3)
and lithium bis[2,3-naphthalenediolato(2−)-O,O ]borate, Li[B(O 2 C 10 H 6 ) 2 ] (4) [211]
of Figure 17.6 show that the underlying oxidation mechanisms are closely related.
There is a difference in the behavior of benzenediolatoborate and
naphthalenediolatoborate solutions on the one hand and lithium
bis[2,2 -biphenyldiolato(2–)-O,O ]borate (5), lithium bis[salicylato(2–)]borate
(6) or benzenediolatoborate/phenolate mixed solutions on the other hand. This
can be tentatively explained by the assumption of different decomposition
mechanisms due to different structures which entail the formation of soluble
colored quinones from benzenediolatoborate anions and lithium-ion conducting
films from solutions of compounds (5) and (6) [107]. The assumption of a
different mechanism and the formation of a lithium-ion conducting electronically
insulating film are supported by:
• The shift of the linear correlation of the values for the highest occupied molecular
orbital E HOMO with anodic decomposition voltages E Ox of about −3eV·V −1 to
higher oxidation potentials by about 1 eV (see Figure 17.6).
6 3
6
5.5
4
−E HOMO eV 5 2 5
1
3.5 3.75 4 4.25 4.5
+
E Ox vs Li/Li
V
Figure 17.6 Linear correlation of HOMO-energies and an-
odic oxidation limits of lithium borates Li[B(C 6 H 4-x F x O 2 ) 2 ],
x = 0(1); x = 1 (2), and x = 4(3),Li[B(O 2 C 10 H 6 ) 2 ](4),
lithium bis[2,2 -biphenyldiolato(2−)-O,O ]borate (5), and
lithium bis[salicylato(2–)]borate (6).