Page 596 - Handbook of Battery Materials
P. 596
570 17 Liquid Nonaqueous Electrolytes
purity, low production cost, and easy accessibility, aluminum is the ideal material
for DLCs and positive electrodes in lithium-ion batteries [284]. Some lithium
salts, for example, LiTFSI and LiOTf, show very good solubility, stability, or
conductivity properties, but react strongly with aluminum, leading to its corrosion
and dissolution [261]. This drawback makes them useless for practical applications
without protecting additives.
If passivation did not take place on the Al surface, electrolyte decomposition and
Al deletion would diminish the cell’s capability and even destroy the whole cell.
But film formation and therefore passivation of the Al surface is highly dependent
on the choice of lithium salt [285] or film-forming additives. Table 17.9 shows
oxidation potentials E Ox of several lithium salts at aluminum.
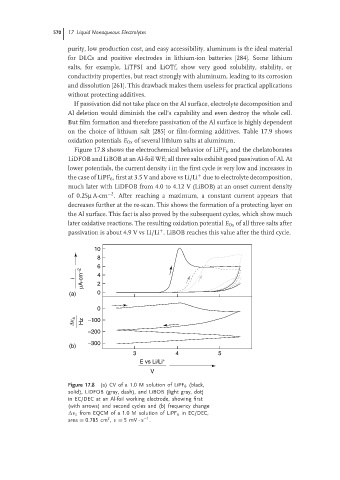
Figure 17.8 shows the electrochemical behavior of LiPF 6 and the chelatoborates
LiDFOB and LiBOB at an Al-foil WE; all three salts exhibit good passivation of Al. At
lower potentials, the current density i in the first cycle is very low and increases in
the case of LiPF 6 ,first at3.5 Vand above vsLi/Li due to electrolyte decomposition,
+
much later with LiDFOB from 4.0 to 4.12 V (LiBOB) at an onset current density
−2
of 0.25µ A·cm . After reaching a maximum, a constant current appears that
decreases further at the re-scan. This shows the formation of a protecting layer on
the Al surface. This fact is also proved by the subsequent cycles, which show much
later oxidative reactions. The resulting oxidation potential E Ox of all three salts after
passivation is about 4.9 V vs Li/Li . LiBOB reaches this value after the third cycle.
+
10
8
6
i µA·cm −2 4 2
(a) 0
0
∆ν s Hz −100
−200
(b) −300
3 4 5
E vs Li/Li +
V
Figure 17.8 (a) CV of a 1.0 M solution of LiPF 6 (black,
solid), LiDFOB (gray, dash), and LiBOB (light gray, dot)
in EC/DEC at an Al-foil working electrode, showing first
(with arrows) and second cycles and (b) frequency change
ν s from EQCM of a 1.0 M solution of LiPF 6 in EC/DEC,
2
−1
area = 0.785 cm , v = 5mV · s .