Page 602 - Handbook of Battery Materials
P. 602
576 17 Liquid Nonaqueous Electrolytes
Table 17.13 Surface reactions of lithium salts at graphite electrodes.
Reaction References
− + [336, 337]
LiAsF 6 + 2e + 2Li → 3LiF ↓+AsF 3
− +
AsF 3 + 2xe + 2xLi → Li x AsF 3−x + xLiF ↓ –
+
−
LiBF 4 + xe + xLi → LiF ↓+ Li x BF y ↓ [339]
− + [336, 337]
LiPF 6 + 2e + 2Li → 3LiF ↓+ PF 3
− +
LiPF 6 + xe + xLi → LiF ↓+ Li x PF y ↓ [333, 339]
[332]
LiPF 6 → LiF + PF 5
LiPF 6 + Li 2 CO 3 → 3LiF + POF 3 + CO 2 –
PF 5 + Li 2 CO 3 → 2LiF + POF 3 + CO 2 –
− [339]
LiCF 3 SO 3 + e → LiF ↓+·CF 2 SO 3
− + [339]
2LiCF 3 SO 3 + 2e + 2Li → 2Li 2 SO 3 ↓+C 2 F 6 ↑
− +
C 2 F 6 + 2e + 2Li → LiCF 2 CF 3 + LiF ↓
− + 1 1
LiC(SO 2 CF 3 ) 3 + ne + nLi → Li 2 S 2 O 4 + mLiF + Li x C 2 F y
2 2
+Li 2 C(SO 2 CF 3 ) 2 [337, 340]
− + [337, 340]
LiC(SO 2 CF 3 ) 3 + 2e + 2Li → Li 2 C(SO 2 CF 3 ) 2 + LiSO 2 CF 3
− + [337, 340]
LiN(SO 2 CF 3 ) 2 + ne + nLi → Li 3 N + Li 2 S 2 O 4 ↓+LiF ↓+Li x C 2 F y
+
−
Li 2 S 2 O 4 + 4e + 4Li → Li 2 SO 3 + Li 2 S ↓+Li 2 O ↓ –
− +
Li 2 S 2 O 4 + 10e + 10Li → 2Li 2 S ↓+4Li 2 O ↓ –
− + [337, 340]
LiN(SO 2 CF 3 ) 2 + 2e + 2Li → Li 2 NSO 2 CF 3 + LiSO 2 CF 3
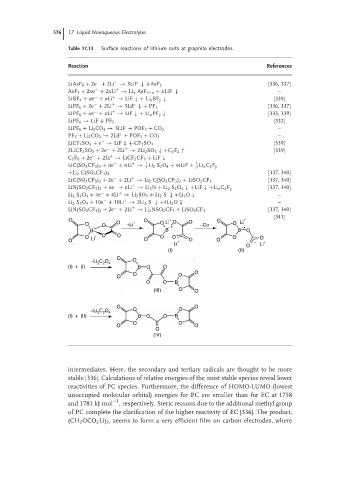
[341]
O + O + O O O +
O O O +Li O Li −Co O Li
− −• −•
B B B O
O Li + O O O O O O
O O + − O O − +
Li O Li
(I) (II)
O
−Li C O 4 O
2
2
(I) + (I) B O O
O O O
O
O O B
(III) O O
O O
C O O O
−Li 2 2 4
(I) + (II) B O O B
O O
O O
O
(IV)
intermediates. Here, the secondary and tertiary radicals are thought to be more
stable [336]. Calculations of relative energies of the most stable species reveal lower
reactivities of PC species. Furthermore, the difference of HOMO-LUMO (lowest
unoccupied molecular orbital) energies for PC are smaller than for EC at 1758
−1
and 1781 kJ·mol , respectively. Steric reasons due to the additional methyl group
of PC complete the clarification of the higher reactivity of EC [336]. The product,
(CH 2 OCO 2 Li) 2 , seems to form a very efficient film on carbon electrodes, where