Page 583 - Handbook of Battery Materials
P. 583
17.3 Intrinsic Properties 557
Table 17.7 (continued)
3
−1
−1
−1
3
2
Solvent Salt K A (dm ·mol ) K T (dm ·mol ) Λ 0 (S cm ·mol ) References
THF LiClO 4 3.7 × 10 7 34 – [212]
THF LiAsF 6 5.3 × 10 5 35 – [212]
THF LiSbF 6 2.7 × 10 5 40.8 – [212]
THF LiBPh 4 2.2 × 10 4 16.5 – [212]
a −45 C.
◦
b ◦
−43 C.
c Equimolar mixtures.
• association constants of the lithium salts strongly depending on the radius of the
anion and its ability to delocalize its charge,
• association constants decreasing strongly with decreasing temperature, and
• association constants decreasing by the substitution of a proton of the anion
Li[B(C 6 H 4 O 2 ) 2 ] by fluorine, entailing increased charge delocalization.
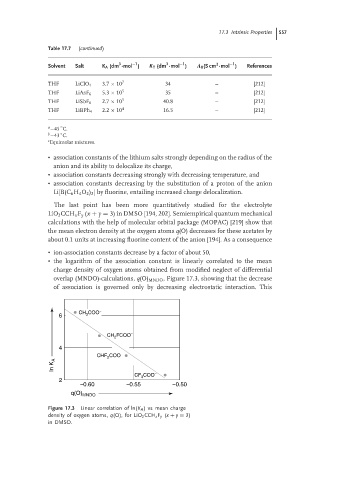
The last point has been more quantitatively studied for the electrolyte
LiO 2 CCH x F y (x + y = 3) in DMSO [194, 202]. Semiempirical quantum mechanical
calculations with the help of molecular orbital package (MOPAC) [219] show that
the mean electron density at the oxygen atoms q(O) decreases for these acetates by
about 0.1 units at increasing fluorine content of the anion [194]. As a consequence
• ion-association constants decrease by a factor of about 50,
• the logarithm of the association constant is linearly correlated to the mean
charge density of oxygen atoms obtained from modified neglect of differential
overlap (MNDO)-calculations, q(O) MNDO , Figure 17.3, showing that the decrease
of association is governed only by decreasing electrostatic interaction. This
−
CH COO
6 3
−
CH FCOO
2
4
−
CHF COO
2
In K A
CF COO −
2 3
−0.60 −0.55 −0.50
q(O) MNDO
Figure 17.3 Linear correlation of ln(K A ) vsmeancharge
density of oxygen atoms, q(O), for LiO 2 CCH x F y (x + y = 3)
in DMSO.