Page 584 - Handbook of Battery Materials
P. 584
558 17 Liquid Nonaqueous Electrolytes
1
6.5
2
5.5
In(K A )
4.5
4.3 4.5 4.7 4.9
4
10 / eT
K −1
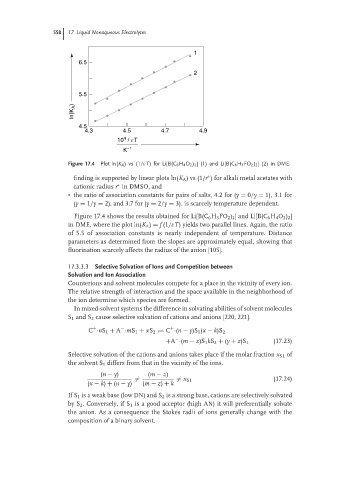
Figure 17.4 Plot ln(K A )vs(1/εT) for Li[B(C 6 H 4 O 2 ) 2 ] (1) and Li[B(C 6 H 3 FO 2 ) 2 ] (2) in DME.
+
finding is supported by linear plots ln(K A )vs (1/r ) for alkali metal acetates with
+
cationic radius r in DMSO, and
• the ratio of association constants for pairs of salts, 4.2 for (y = 0/y = 1), 3.1 for
(y = 1/y = 2), and 3.7 for (y = 2/y = 3), is scarcely temperature dependent.
Figure 17.4 shows the results obtained for Li[B(C 6 H 3 FO 2 ) 2 ] and Li[B(C 6 H 4 O 2 ) 2 ]
in DME, where the plot ln(K A ) = f (1/εT) yields two parallel lines. Again, the ratio
of 5.5 of association constants is nearly independent of temperature. Distance
parameters as determined from the slopes are approximately equal, showing that
fluorination scarcely affects the radius of the anion [105].
17.3.3.3 Selective Solvation of Ions and Competition between
Solvation and Ion Association
Counterions and solvent molecules compete for a place in the vicinity of every ion.
The relative strength of interaction and the space available in the neighborhood of
the ion determine which species are formed.
In mixed-solvent systems the difference in solvating abilities of solvent molecules
S 1 and S 2 cause selective solvation of cations and anions [220, 221].
+ − +
C ·nS 1 + A ·mS 1 + xS 2 C ·(n − y)S 1 (x − k)S 2
−
+A ·(m − z)S 1 kS 2 + (y + z)S 1 (17.23)
Selective solvation of the cations and anions takes place if the molar fraction x S1 of
the solvent S 1 differs from that in the vicinity of the ions.
(n − y) (m − z)
=
= x S1 (17.24)
(x − k) + (n − y) (m − z) + k
If S 1 is a weak base (low DN) and S 2 is a strong base, cations are selectively solvated
by S 2 . Conversely, if S 1 is a good acceptor (high AN) it will preferentially solvate
the anion. As a consequence the Stokes radii of ions generally change with the
composition of a binary solvent.