Page 610 - Handbook of Battery Materials
P. 610
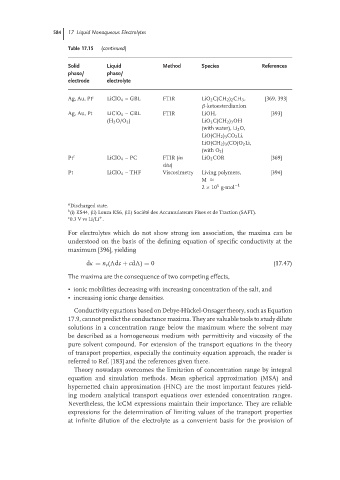
584 17 Liquid Nonaqueous Electrolytes
Table 17.15 (continued)
Solid Liquid Method Species References
phase/ phase/
electrode electrolyte
Ag, Au, Pt c LiClO 4 – GBL FTIR LiO 2 C(CH 2 ) 2 CH 3 , [369, 393]
β-ketoesterdianion
Ag, Au, Pt LiClO 4 –GBL FTIR LiOH, [393]
(H 2 O/O 2 ) LiO 2 C(CH 2 ) 3 OH
(with water), Li 2 O,
LiO(CH 2 ) 3 CO 2 Li,
LiO(CH 2 ) 3 (CO)O 2 Li,
(with O 2 )
Pt c LiClO 4 –PC FTIR (in LiO 2 COR [369]
situ)
Pt LiClO 4 – THF Viscosimetry Living polymers, [394]
M ≈
5
2 × 10 g·mol −1
a Discharged state.
b
(i) KS44, (ii) Lonza KS6, (iii) Soci´ et´ e des Accumulateurs Fixes et de Traction (SAFT).
c 0.3 V vs Li/Li .
+
For electrolytes which do not show strong ion association, the maxima can be
understood on the basis of the defining equation of specific conductivity at the
maximum [396], yielding
dκ = n e ( dc + cd ) = 0 (17.47)
The maxima are the consequence of two competing effects,
• ionic mobilities decreasing with increasing concentration of the salt, and
• increasing ionic charge densities.
Conductivity equations based on Debye-H¨ uckel-Onsager theory, such as Equation
17.9, cannot predict the conductance maxima. They are valuable tools to study dilute
solutions in a concentration range below the maximum where the solvent may
be described as a homogeneous medium with permittivity and viscosity of the
pure solvent compound. For extension of the transport equations in the theory
of transport properties, especially the continuity equation approach, the reader is
referred to Ref. [183] and the references given there.
Theory nowadays overcomes the limitation of concentration range by integral
equation and simulation methods. Mean spherical approximation (MSA) and
hypernetted chain approximation (HNC) are the most important features yield-
ing modern analytical transport equations over extended concentration ranges.
Nevertheless, the lcCM expressions maintain their importance. They are reliable
expressions for the determination of limiting values of the transport properties
at infinite dilution of the electrolyte as a convenient basis for the provision of