Page 62 - Handbook of Battery Materials
P. 62
28 2 Practical Batteries
progress in alkaline-zinc batteries and manufactured zinc powder with high surface
area to prevent zinc passivation.
The discharge of alkaline-manganese batteries comes from the electrochemical
reactions at the anode and cathode. During discharge, the negative electrode
material, zinc, is oxidized, forming zinc oxide; at the same time, MnO 2 in the
positive electrode is reduced (MnOOH):
Cathode reaction:
2MnO 2 + H 2 O + 2e → 2MnOOH + 2OH − 0.12 V vs NHE (2.1)
−
Anode reaction:
Zn + 2OH → ZnO + H 2 O + 2e − − 1.33 V vs NHE (2.2)
−
Overall reaction:
Zn + 2MnO 2 → ZnO + 2MnOOH 1.45 V (2.3)
The initial voltage of an alkaline-manganese dioxide battery is about 1.5 V.
Alkaline-manganese batteries use a concentrated alkaline aqueous solution (typ-
ically in the range of 30–45% potassium hydroxide) for electrolyte. In this
concentrated electrolyte, the zinc electrode reaction proceeds, but if the con-
centration of the alkaline solution is low, then the zinc tends to passivate.
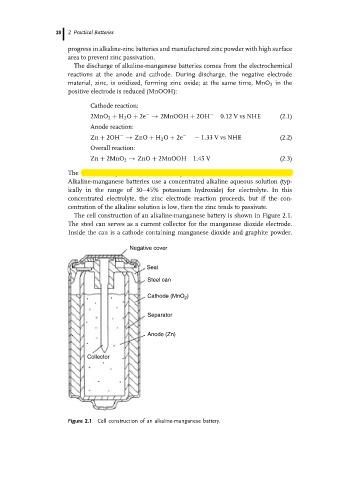
The cell construction of an alkaline-manganese battery is shown in Figure 2.1.
The steel can serves as a current collector for the manganese dioxide electrode.
Inside the can is a cathode containing manganese dioxide and graphite powder.
Negative cover
Seal
Steel can
Cathode (MnO )
2
Separator
Anode (Zn)
Collector
Figure 2.1 Cell construction of an alkaline-manganese battery.