Page 64 - Handbook of Battery Materials
P. 64
30 2 Practical Batteries
powder with indium, bismuth, and other additives [1–3]. Adding indium to zinc
powder is the most effective way to improve the characteristics of the cells [2].
Figure 2.3 shows the variation in the internal impedance of the cells according to
the additive content of the zinc powder.
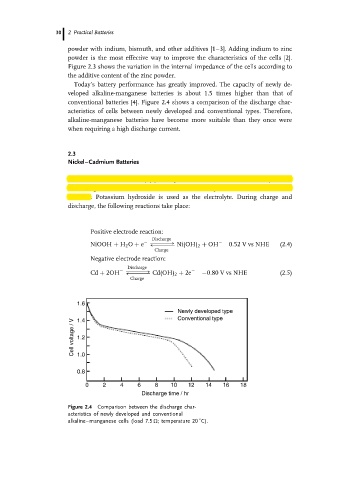
Today’s battery performance has greatly improved. The capacity of newly de-
veloped alkaline-manganese batteries is about 1.5 times higher than that of
conventional batteries [4]. Figure 2.4 shows a comparison of the discharge char-
acteristics of cells between newly developed and conventional types. Therefore,
alkaline-manganese batteries have become more suitable than they once were
when requiring a high discharge current.
2.3
Nickel–Cadmium Batteries
The nickel–cadmium battery [5] has a positive electrode made of nickel hydroxide
and a negative electrode in which a cadmium compound is used as the active
material. Potassium hydroxide is used as the electrolyte. During charge and
discharge, the following reactions take place:
Positive electrode reaction:
Discharge
NiOOH + H 2 O + e −−−−−−→ Ni(OH) 2 + OH − 0.52 V vs NHE (2.4)
−
←−−−−−−
Charge
Negative electrode reaction:
Discharge
−
Cd + 2OH −−−−−−→ Cd(OH) 2 + 2e − −0.80 V vs NHE (2.5)
←−−−−−−
Charge
1.6
Newly developed type
Conventional type
Cell voltage / V 1.2
1.4
1.0
0.8
0 2 4 6 8 10 12 14 16 18
Discharge time / hr
Figure 2.4 Comparison between the discharge char-
acteristics of newly developed and conventional
◦
alkaline–manganese cells (load 7.5 ; temperature 20 C).