Page 68 - Handbook of Battery Materials
P. 68
34 2 Practical Batteries
100
Discharge Capacity (%) 60 Cycle conditions
80
Charging:0.1C×11Hrs.
Discharge:0.7C×1Hrs.
Temperature:20°C
40
Capacity measuring conditions
Charging:0.1C×16Hrs.
Discharge:0.2C, E.V.=0.1V
20
Temperature:20°C
0
0 200 400 600 800 1000
Number of cycles
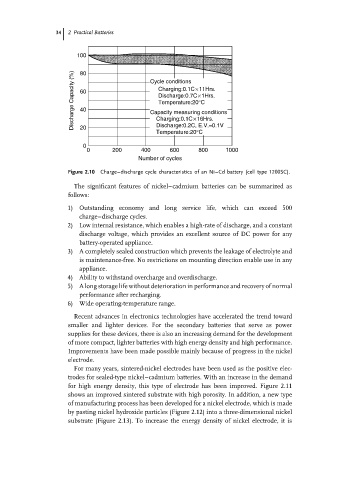
Figure 2.10 Charge–discharge cycle characteristics of an Ni–Cd battery (cell type 1200SC).
The significant features of nickel–cadmium batteries can be summarized as
follows:
1) Outstanding economy and long service life, which can exceed 500
charge–discharge cycles.
2) Low internal resistance, which enables a high-rate of discharge, and a constant
discharge voltage, which provides an excellent source of DC power for any
battery-operated appliance.
3) A completely sealed construction which prevents the leakage of electrolyte and
is maintenance-free. No restrictions on mounting direction enable use in any
appliance.
4) Ability to withstand overcharge and overdischarge.
5) A long storage life without deterioration in performance and recovery of normal
performance after recharging.
6) Wide operating-temperature range.
Recent advances in electronics technologies have accelerated the trend toward
smaller and lighter devices. For the secondary batteries that serve as power
supplies for these devices, there is also an increasing demand for the development
of more compact, lighter batteries with high energy density and high performance.
Improvements have been made possible mainly because of progress in the nickel
electrode.
For many years, sintered-nickel electrodes have been used as the positive elec-
trodes for sealed-type nickel–cadmium batteries. With an increase in the demand
for high energy density, this type of electrode has been improved. Figure 2.11
shows an improved sintered substrate with high porosity. In addition, a new type
of manufacturing process has been developed for a nickel electrode, which is made
by pasting nickel hydroxide particles (Figure 2.12) into a three-dimensional nickel
substrate (Figure 2.13). To increase the energy density of nickel electrode, it is