Page 71 - Handbook of Battery Materials
P. 71
2.4 Nickel–MH Batteries 37
Charge Discharge
− −
OH NiOOH OH NiOOH
+ +
H H
H H
H O Ni (OH) 2 H O Ni (OH) 2
2
2
MH+NiOOH MH+NiOOH M+Ni (OH)
M+Ni (OH) 2 2
:hydrogen absorbing alloy
:hydrogen
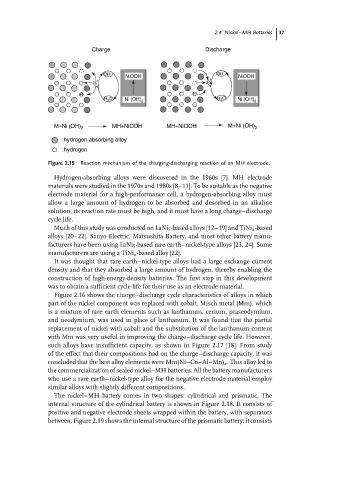
Figure 2.15 Reaction mechanism of the charging-discharging reaction of an MH electrode.
Hydrogen-absorbing alloys were discovered in the 1960s [7]. MH electrode
materials were studied in the 1970s and 1980s [8–11]. To be suitable as the negative
electrode material for a high-performance cell, a hydrogen-absorbing alloy must
allow a large amount of hydrogen to be absorbed and desorbed in an alkaline
solution, its reaction rate must be high, and it must have a long charge–discharge
cycle life.
Much of this study was conducted on LaNi 5 -based alloys [12–19] and TiNi x -based
alloys [20–22]. Sanyo Electric, Matsushita Battery, and most other battery manu-
facturers have been using LaNi 5 -based rare earth–nickel-type alloys [23, 24]. Some
manufacturers are using a TiNi x -based alloy [22].
It was thought that rare earth–nickel-type alloys had a large exchange current
density and that they absorbed a large amount of hydrogen, thereby enabling the
construction of high-energy-density batteries. The first step in this development
was to obtain a sufficient cycle life for their use as an electrode material.
Figure 2.16 shows the charge–discharge cycle characteristics of alloys in which
part of the nickel component was replaced with cobalt. Misch metal (Mm), which
is a mixture of rare earth elements such as lanthanum, cerium, praseodymium,
and neodymium, was used in place of lanthanum. It was found that the partial
replacement of nickel with cobalt and the substitution of the lanthanum content
with Mm was very useful in improving the charge–discharge cycle life. However,
such alloys have insufficient capacity, as shown in Figure 2.17 [18]. From study
of the effect that their compositions had on the charge–discharge capacity, it was
concluded that the best alloy elements were Mm(Ni–Co–Al–Mn) x . This alloy led to
the commercialization of sealed nickel–MH batteries. All the battery manufacturers
who use a rare earth–nickel-type alloy for the negative electrode material employ
similar alloys with slightly different compositions.
The nickel–MH battery comes in two shapes: cylindrical and prismatic. The
internal structure of the cylindrical battery is shown in Figure 2.18. It consists of
positive and negative electrode sheets wrapped within the battery, with separators
between. Figure 2.19 shows the internal structure of the prismatic battery: it consists