Page 75 - Handbook of Battery Materials
P. 75
2.4 Nickel–MH Batteries 41
1.6
Charging:0.1×16h
Rest:1h
1.4 0.2C Temperature:25 °C
Battery voltage (V) 1.2 1C
1.0
3C
0.8
0.6
0 20 40 60 80 100 120
Discharge capacity (%)
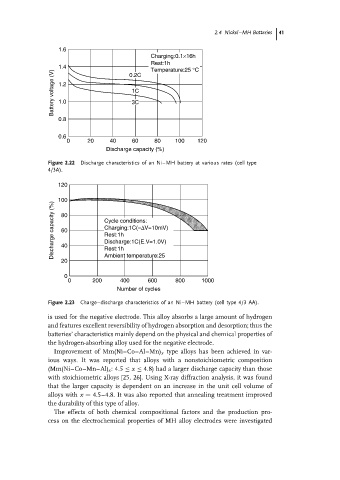
Figure 2.22 Discharge characteristics of an Ni–MH battery at various rates (cell type
4/3A).
120
100
Discharge capacity (%) 60 Cycle conditions:
80
Charging:1C(–∆V=10mV)
Rest:1h
Discharge:1C(E.V=1.0V)
40
Ambient temperature:25
20 Rest:1h
0
0 200 400 600 800 1000
Number of cycles
Figure 2.23 Charge–discharge characteristics of an Ni–MH battery (cell type 4/3 AA).
is used for the negative electrode. This alloy absorbs a large amount of hydrogen
and features excellent reversibility of hydrogen absorption and desorption; thus the
batteries’ characteristics mainly depend on the physical and chemical properties of
the hydrogen-absorbing alloy used for the negative electrode.
Improvement of Mm(Ni–Co–Al–Mn) x type alloys has been achieved in var-
ious ways. It was reported that alloys with a nonstoichiometric composition
(Mm(Ni–Co–Mn–Al) x :4.5 ≤ x ≤ 4.8) had a larger discharge capacity than those
with stoichiometric alloys [25, 26]. Using X-ray diffraction analysis, it was found
that the larger capacity is dependent on an increase in the unit cell volume of
alloys with x = 4.5–4.8. It was also reported that annealing treatment improved
the durability of this type of alloy.
The effects of both chemical compositional factors and the production pro-
cess on the electrochemical properties of MH alloy electrodes were investigated