Page 153 - Handbook of Energy Engineering Calculations
P. 153
2
14.9 lb/in (abs), with an engine efficiency of 85 percent. Assuming that the
combustion products have the same thermodynamic properties as air, c =
p
0.24, and is constant. The isentropic exponent may be taken as 1.4. (a) Find
the temperature after compression, after combustion, and at the exhaust; (b)
Determine the Btu/lb (kJ/kg) of air supplied, the work delivered by the
expander, the net work produced by the gas turbine, and its thermal
efficiency.
Calculation Procedure:
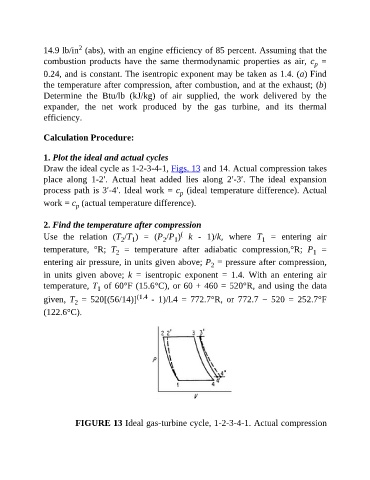
1. Plot the ideal and actual cycles
Draw the ideal cycle as 1-2-3-4-1, Figs. 13 and 14. Actual compression takes
place along 1-2′. Actual heat added lies along 2′-3′. The ideal expansion
process path is 3′-4′. Ideal work = c (ideal temperature difference). Actual
p
work = c (actual temperature difference).
p
2. Find the temperature after compression
(
Use the relation (T /T ) = (P /P ) k - 1)/k, where T = entering air
1
1
2
1
2
temperature, °R; T = temperature after adiabatic compression,°R; P =
2
1
entering air pressure, in units given above; P = pressure after compression,
2
in units given above; k = isentropic exponent = 1.4. With an entering air
temperature, T of 60°F (15.6°C), or 60 + 460 = 520°R, and using the data
1
given, T = 520[(56/14)] (1.4 - 1)/l.4 = 772.7°R, or 772.7 − 520 = 252.7°F
2
(122.6°C).
FIGURE 13 Ideal gas-turbine cycle, 1-2-3-4-1. Actual compression