Page 152 - Handbook of Plastics Technologies
P. 152
THERMOSETS
3.22 CHAPTER 3
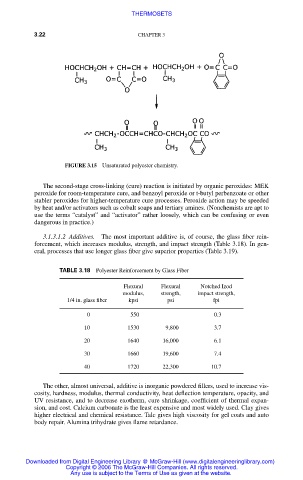
FIGURE 3.15 Unsaturated polyester chemistry.
The second-stage cross-linking (cure) reaction is initiated by organic peroxides: MEK
peroxide for room-temperature cure, and benzoyl peroxide or t-butyl perbenzoate or other
stabler peroxides for higher-temperature cure processes. Peroxide action may be speeded
by heat and/or activators such as cobalt soaps and tertiary amines. (Nonchemists are apt to
use the terms “catalyst” and “activator” rather loosely, which can be confusing or even
dangerous in practice.)
3.1.3.1.2 Additives. The most important additive is, of course, the glass fiber rein-
forcement, which increases modulus, strength, and impact strength (Table 3.18). In gen-
eral, processes that use longer glass fiber give superior properties (Table 3.19).
TABLE 3.18 Polyester Reinforcement by Glass Fiber
Flexural Flexural Notched Izod
modulus, strength, impact strength,
1/4 in. glass fiber kpsi psi fpi
0 550 0.3
10 1530 9,800 3.7
20 1640 16,000 6.1
30 1660 19,600 7.4
40 1720 22,300 10.7
The other, almost universal, additive is inorganic powdered fillers, used to increase vis-
cosity, hardness, modulus, thermal conductivity, heat deflection temperature, opacity, and
UV resistance, and to decrease exotherm, cure shrinkage, coefficient of thermal expan-
sion, and cost. Calcium carbonate is the least expensive and most widely used. Clay gives
higher electrical and chemical resistance. Talc gives high viscosity for gel coats and auto
body repair. Alumina trihydrate gives flame retardance.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.