Page 147 - Handbook of Plastics Technologies
P. 147
THERMOSETS
THERMOSETS 3.17
TABLE 3.13 Urea-Formaldehyde Moldings: Typical
Properties
Specific gravity 1.5
Tensile modulus 1,300,000 psi
Flexural modulus 1,450,000 psi
Tensile strength 8,250 psi
Flexural strength 13,000 psi
Impact strength 0.31 fpi
–6 o
Thermal expansion 29 × 10 / C
o
Heat deflection temperature 133 C
Dielectric constant 6.8
14
Volume resistivity 10 Ω-cm
Water absorption 0.6%
3.1.2.3 Melamine-Formaldehyde. Melamine-formaldehyde and urea-formaldehyde
have similar polymerization chemistry, so they are often referred to as “amino resins.”
However, they differ in properties, applications, economics, and market volume, so they
are best studied independently. Melamine offers superior resistance to heat, weather, and
moisture, but it is more expensive than urea, so it is used only when its superior perfor-
mance is required. The U.S. market volume is about 350 million lb/yr.
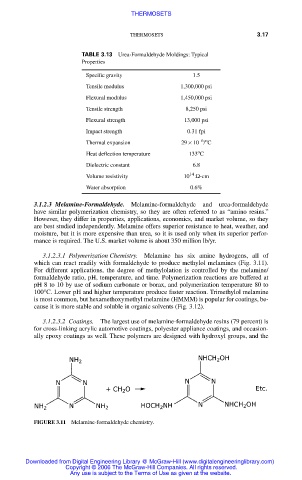
3.1.2.3.1 Polymerization Chemistry. Melamine has six amine hydrogens, all of
which can react readily with formaldehyde to produce methylol melamines (Fig. 3.11).
For different applications, the degree of methylolation is controlled by the melamine/
formaldehyde ratio, pH, temperature, and time. Polymerization reactions are buffered at
pH 8 to 10 by use of sodium carbonate or borax, and polymerization temperature 80 to
100°C. Lower pH and higher temperature produce faster reaction. Trimethylol melamine
is most common, but hexamethoxymethyl melamine (HMMM) is popular for coatings, be-
cause it is more stable and soluble in organic solvents (Fig. 3.12).
3.1.2.3.2 Coatings. The largest use of melamine-formaldehyde resins (79 percent) is
for cross-linking acrylic automotive coatings, polyester appliance coatings, and occasion-
ally epoxy coatings as well. These polymers are designed with hydroxyl groups, and the
FIGURE 3.11 Melamine-formaldehyde chemistry.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.