Page 157 - Handbook of Plastics Technologies
P. 157
THERMOSETS
THERMOSETS 3.27
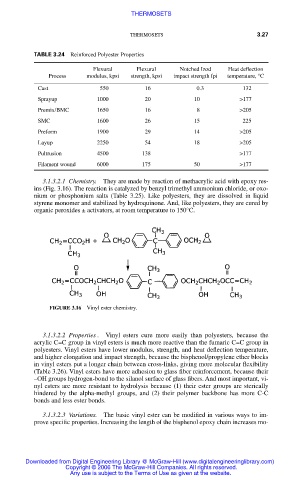
TABLE 3.24 Reinforced Polyester Properties
Flexural Flexural Notched Izod Heat deflection
Process modulus, kpsi strength, kpsi impact strength fpi temperature, °C
Cast 550 16 0.3 132
Sprayup 1000 20 10 >177
Premix/BMC 1650 16 8 >205
SMC 1600 26 15 225
Preform 1900 29 14 >205
Layup 2250 54 18 >205
Pultrusion 4500 138 >177
Filament wound 6000 175 50 >177
3.1.3.2.1 Chemistry. They are made by reaction of methacrylic acid with epoxy res-
ins (Fig. 3.16). The reaction is catalyzed by benzyl trimethyl ammonium chloride, or oxo-
nium or phosphonium salts (Table 3.25). Like polyesters, they are dissolved in liquid
styrene monomer and stabilized by hydroquinone. And, like polyesters, they are cured by
organic peroxides ± activators, at room temperature to 150°C.
FIGURE 3.16 Vinyl ester chemistry.
3.1.3.2.2 Properties . Vinyl esters cure more easily than polyesters, because the
acrylic C=C group in vinyl esters is much more reactive than the fumaric C=C group in
polyesters. Vinyl esters have lower modulus, strength, and heat deflection temperature,
and higher elongation and impact strength, because the bisphenol/propylene ether blocks
in vinyl esters put a longer chain between cross-links, giving more molecular flexibility
(Table 3.26). Vinyl esters have more adhesion to glass fiber reinforcement, because their
–OH groups hydrogen-bond to the silanol surface of glass fibers. And most important, vi-
nyl esters are more resistant to hydrolysis because (1) their ester groups are sterically
hindered by the alpha-methyl groups, and (2) their polymer backbone has more C-C
bonds and less ester bonds.
3.1.3.2.3 Variations. The basic vinyl ester can be modified in various ways to im-
prove specific properties. Increasing the length of the bisphenol epoxy chain increases mo-
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.