Page 161 - Handbook of Plastics Technologies
P. 161
THERMOSETS
THERMOSETS 3.31
in other polymerization and cure reactions. Choice of a range of epoxy monomers and cur-
ing agents, as well as additives, leads to a wide range of final properties for different appli-
cations.
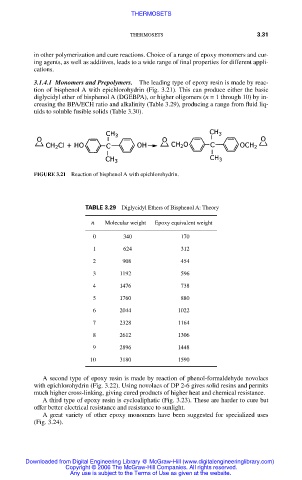
3.1.4.1 Monomers and Prepolymers. The leading type of epoxy resin is made by reac-
tion of bisphenol A with epichlorohydrin (Fig. 3.21). This can produce either the basic
diglycidyl ether of bisphenol A (DGEBPA), or higher oligomers (n = 1 through 10) by in-
creasing the BPA/ECH ratio and alkalinity (Table 3.29), producing a range from fluid liq-
uids to soluble fusible solids (Table 3.30).
FIGURE 3.21 Reaction of bisphenol A with epichlorohydrin.
TABLE 3.29 Diglycidyl Ethers of Bisphenol A: Theory
n Molecular weight Epoxy equivalent weight
0 340 170
1 624 312
2 908 454
3 1192 596
4 1476 738
5 1760 880
6 2044 1022
7 2328 1164
8 2612 1306
9 2896 1448
10 3180 1590
A second type of epoxy resin is made by reaction of phenol-formaldehyde novolacs
with epichlorohydrin (Fig. 3.22). Using novolacs of DP 2-6 gives solid resins and permits
much higher cross-linking, giving cured products of higher heat and chemical resistance.
A third type of epoxy resin is cycloaliphatic (Fig. 3.23). These are harder to cure but
offer better electrical resistance and resistance to sunlight.
A great variety of other epoxy monomers have been suggested for specialized uses
(Fig. 3.24).
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.