Page 160 - Handbook of Plastics Technologies
P. 160
THERMOSETS
3.30 CHAPTER 3
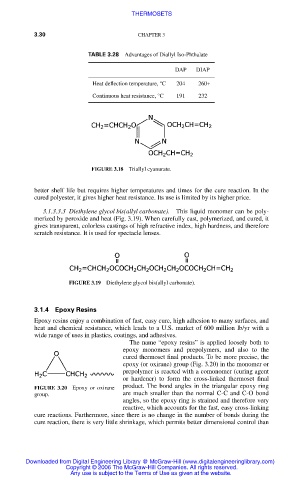
TABLE 3.28 Advantages of Diallyl Iso-Phthalate
DAP DIAP
Heat deflection temperature, °C 204 260+
Continuous heat resistance, °C 191 232
FIGURE 3.18 Triallyl cyanurate.
better shelf life but requires higher temperatures and times for the cure reaction. In the
cured polyester, it gives higher heat resistance. Its use is limited by its higher price.
3.1.3.3.3 Diethylene glycol bis(allyl carbonate). This liquid monomer can be poly-
merized by peroxide and heat (Fig. 3.19). When carefully cast, polymerized, and cured, it
gives transparent, colorless castings of high refractive index, high hardness, and therefore
scratch resistance. It is used for spectacle lenses.
FIGURE 3.19 Diethylene glycol bis(allyl carbonate).
3.1.4 Epoxy Resins
Epoxy resins enjoy a combination of fast, easy cure, high adhesion to many surfaces, and
heat and chemical resistance, which leads to a U.S. market of 600 million lb/yr with a
wide range of uses in plastics, coatings, and adhesives.
The name “epoxy resins” is applied loosely both to
epoxy monomers and prepolymers, and also to the
cured thermoset final products. To be more precise, the
epoxy (or oxirane) group (Fig. 3.20) in the monomer or
prepolymer is reacted with a comonomer (curing agent
or hardener) to form the cross-linked thermoset final
FIGURE 3.20 Epoxy or oxirane product. The bond angles in the triangular epoxy ring
group. are much smaller than the normal C-C and C-O bond
angles, so the epoxy ring is strained and therefore very
reactive, which accounts for the fast, easy cross-linking
cure reactions. Furthermore, since there is no change in the number of bonds during the
cure reaction, there is very little shrinkage, which permits better dimensional control than
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.