Page 375 - Handbook of Properties of Textile and Technical Fibres
P. 375
348 Handbook of Properties of Textile and Technical Fibres
In the 1970s, collagen molecule was thought to be a rigid rod based on the obser-
2
vation that the translational diffusion constant was 0.86 10 6 cm /s, which was very
close to that calculated for a prolate ellipsoid 1.5 nm wide and 300 nm long (Silver
et al., 1979). However, later measurements, based on rotary shadowed images of
collagen molecules, suggested that the molecules had numerous bends as shown in
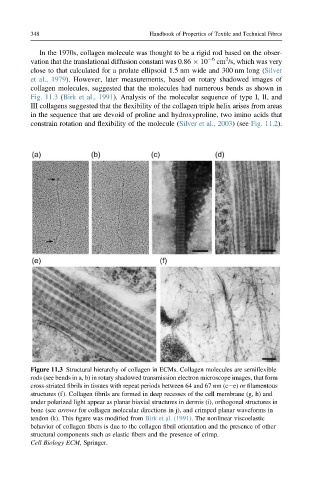
Fig. 11.3 (Birk et al., 1991). Analysis of the molecular sequence of type I, II, and
III collagens suggested that the flexibility of the collagen triple helix arises from areas
in the sequence that are devoid of proline and hydroxyproline, two imino acids that
constrain rotation and flexibility of the molecule (Silver et al., 2003) (see Fig. 11.2).
Figure 11.3 Structural hierarchy of collagen in ECMs. Collagen molecules are semiflexible
rods (see bends in a, b) in rotary shadowed transmission electron microscope images, that form
cross-striated fibrils in tissues with repeat periods between 64 and 67 nm (cee) or filamentous
structures (f). Collagen fibrils are formed in deep recesses of the cell membrane (g, h) and
under polarized light appear as planar biaxial structures in dermis (i), orthogonal structures in
bone (see arrows for collagen molecular directions in j), and crimped planar waveforms in
tendon (k). This figure was modified from Birk et al. (1991). The nonlinear viscoelastic
behavior of collagen fibers is due to the collagen fibril orientation and the presence of other
structural components such as elastic fibers and the presence of crimp.
Cell Biology ECM, Springer.