Page 376 - Handbook of Properties of Textile and Technical Fibres
P. 376
Structure and behavior of collagen fibers 349
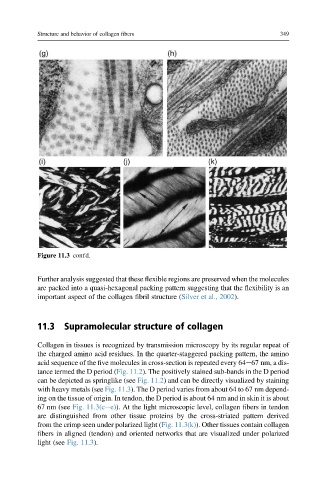
Figure 11.3 cont'd.
Further analysis suggested that these flexible regions are preserved when the molecules
are packed into a quasi-hexagonal packing pattern suggesting that the flexibility is an
important aspect of the collagen fibril structure (Silver et al., 2002).
11.3 Supramolecular structure of collagen
Collagen in tissues is recognized by transmission microscopy by its regular repeat of
the charged amino acid residues. In the quarter-staggered packing pattern, the amino
acid sequence of the five molecules in cross-section is repeated every 64e67 nm, a dis-
tance termed the D period (Fig. 11.2). The positively stained sub-bands in the D period
can be depicted as springlike (see Fig. 11.2) and can be directly visualized by staining
with heavy metals (see Fig. 11.3). The D period varies from about 64 to 67 nm depend-
ing on the tissue of origin. In tendon, the D period is about 64 nm and in skin it is about
67 nm (see Fig. 11.3(cee)). At the light microscopic level, collagen fibers in tendon
are distinguished from other tissue proteins by the cross-striated pattern derived
from the crimp seen under polarized light (Fig. 11.3(k)). Other tissues contain collagen
fibers in aligned (tendon) and oriented networks that are visualized under polarized
light (see Fig. 11.3).