Page 42 - Handbook of Surface Improvement and Modification
P. 42
2.4 Properties and Application data 37
loidal silica topcoat have excellent performance because of high hardness which improves
60
mar resistance.
Sol-gel based UV cured hybrid coatings contained silanes with various urethane
61
acrylate monomers. A lower acrylic silane content and higher inorganic/organic weight
61
ratio improved hardness, elastic modulus and scratch resistance.
The polyurethane coating deposited on the highly ductile and flexible polycarbonate
62
was softer (lower scratch and plowing hardness). The underlying layer of polycarbonate
was able to relieve a large part of the imposed stress during scratching procedures causing
62
delay in the onset of failure.
The subsequent layers having high affinity to the surrounding materials were formu-
lated and deposited on polycarbonate by low cost manufacturing routes, suitable for large-
63
scale industrial applications. The idea of this design (so-called Lego-design) is explained
63
in Figure 2.27 and the chemistry of coating is shown in Figure 2.28. The 3,4-epoxycy-
clohexylmethyl 3,4-epoxycyclohexanecarboxylate resin (primer) adheres to polycarbon-
63
ate due to the reaction of oxirane rings with some free hydroxyl groups in polymer. The
bridging layer, a mixture of AMEO and MEMO organo-silanes, forms covalent bonds
63
with primer. The acrylate-based topcoat forms the covalent bonds between the acrylate
63
groups in the topcoat and MEMO in the bridging layer. Scratch resistance of the coated
63
polycarbonate is increased significantly.
The automotive coatings are one of the examples of anti-corrosive coatings. It is
apparent from the above examples that for a coating to perform its anticorrosive function,
it must first remain intact if subjected to mechanical forces such as scratching and impact
forces and the effect of weather conditions. Below we give some examples of anticorro-
sive coatings.
Anticorrosion coatings pro-
vide barrier functionality against
the corrosive species present in
64
the environment. Temperature,
humidity, and the presence of
aggressive chemicals may cause
the formation of defects and
micropores that deteriorate the
barrier effect and initiate corro-
64
sion. The macrosized particles
present in the traditional coatings
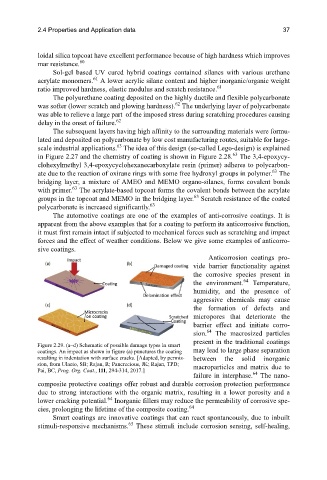
Figure 2.29. (a–d) Schematic of possible damage types in smart
coatings. An impact as shown in figure (a) punctures the coating may lead to large phase separation
resulting in indentation with surface cracks. [Adapted, by permis- between the solid inorganic
sion, from Ulaeto, SB; Rajan, R; Pancrecious, JK; Rajan, TPD; macroparticles and matrix due to
Pai, BC, Prog. Org. Coat., 111, 294-314, 2017.]
64
failure in interphase. The nano-
composite protective coatings offer robust and durable corrosion protection performance
due to strong interactions with the organic matrix, resulting in a lower porosity and a
64
lower cracking potential. Inorganic fillers may reduce the permeability of corrosive spe-
64
cies, prolonging the lifetime of the composite coating.
Smart coatings are innovative coatings that can react spontaneously, due to inbuilt
65
stimuli-responsive mechanisms. These stimuli include corrosion sensing, self-healing,