Page 179 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 179
156 High Temperature Solid Oxide Fuel CelZs: Fundamentals, Design and Applications
6.5 Anode Behaviour Under Steady-State Conditions
The details of the electrode reactions and associated polarisations are discussed
in Chapter 9. In this and the next two sections, anode behaviour under various
conditions is described.
Having formed the anode onto the electrolyte, it is necessary to test its
electrochemical performance and to compare it with the electrolyte, cathode and
interconnect contributions to the overall cell resistance. The characteristics of the
individual electrodes, anode or cathode, and their interfaces with the electrolyte
are dBicult to distinguish from the behaviour of the fuel cell as a whole. In
standard liquid-phase electrochemistry this can be done by use of a third reference
electrode, with respect to which the potential of the electrode under investigation
can be determined. In the fuel cell case the geometry and location of the reference
contact can introduce experimental artefacts, complicating the investigation of
the electrode characteristics [15]. Also since the Nernst equation predicts an
open-circuit potential difference between the electrodes dependent on the oxygen
partial pressure on the anode side, it is frequently advisable to monitor potentials
generally in the cell using a cathode-side reference the potential of which is
therefore fixed by exposure to air. Despite these difficulties, d.c. measurement
using the three-electrode configuration is the best steady-state evaluation
technique which has given valuable insights into anodic processes.
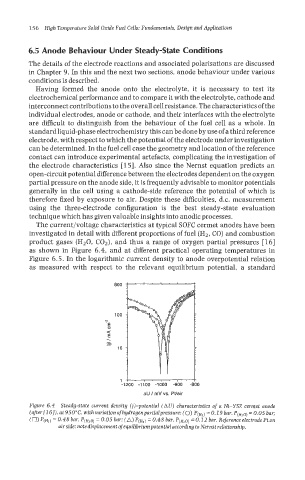
The current/voltage characteristics at typical SOFC cermet anodes have been
investigated in detail with different proportions of fuel (Hz, CO) and combustion
product gases (H20, COZ), and thus a range of oxygen partial pressures [16]
as shown in Figure 6.4, and at different practical operating temperatures in
Figure 6.5. In the logarithmic current density to anode overpotential relation
as measured with respect to the relevant equiIibrium potential, a standard
- 1
-1200 -1100 -1000 -900 4 '0
AU 1 mV vs. Pt/air
Figure 6.4 Steady-state current density (j)-potential (AU) characteristics of a Ni-YSZ cermet anode
(after[163), at 95O"C, with variation ofhydrogenpartialpressure: (0) Pp2) = 0.1 9 bar, P(H~O) = 0.05 bar;
(0) Pp2) = 0.48 bar, P(H~o) 0.05 bar; (A) P(H~) 0.48 bar, P(H,o) = 0.12 bar. Reference electrodept on
=
=
air side; note displacement of equilibriumpotential according to Nernst relationship.