Page 184 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 184
Anodes 161
I I I I I I
0 2 4 6 8 10
z' [QI
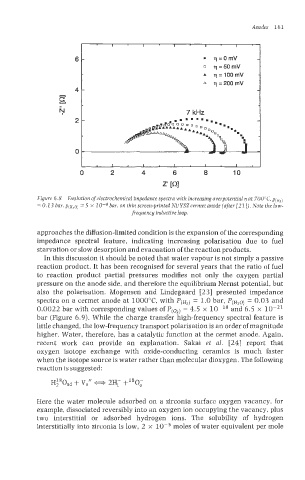
Figure 6.8 Evolution ofelectrochemical impedance spectra with increasing overpotential q at 700"C, p(~>)
5
= 0.13 bar, ~(H~Q x 1 0-4 bar, on thin screen-printed NijYSZ cermet anode (after /21 I). Note the low-
=
frequencg inductive loop.
approaches the diffusion-limited condition is the expansion of the corresponding
impedance spectral feature, indicating increasing polarisation due to fuel
starvation or slow desorption and evacuation of the reaction products.
In this discussion it should be noted that water vapour is not simply a passive
reaction product. It has been recognised for several years that the ratio of fuel
to reaction product partial pressures modifies not only the oxygen partial
pressure on the anode side, and therefore the equilibrium Nernst potential, but
also the polarisation. Mogensen and Lindegaard [2 31 presented impedance
spectra on a cermet anode at 1000°C, with Pp2) = 1.0 bar, Pp20) = 0.03 and
0.0022 bar with corresponding values of P(02) = 4.5 x and 6.5 x
bar (Figure 6.9). While the charge transfer high-frequency spectral feature is
little changed, the low-frequency transport polarisation is an order of magnitude
higher. Water, therefore, has a catalytic function at the cermet anode. Again,
recent work can provide an explanation. SaItai et al. E241 report that
oxygen isotope exchange with oxide-conducting ceramics is much faster
when the isotope source is water rather than molecular dioxygen. The following
reaction is suggested:
Here the water molecule adsorbed on a zirconia surface oxygen vacancy, for
example, dissociated reversibly into an oxygen ion occupying the vacancy, plus
two interstitial or adsorbed hydrogen ions. The solubility of hydrogen
interstitially into zirconia is low, 2 x lo-' moles of water equivalent per mole