Page 187 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 187
164 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
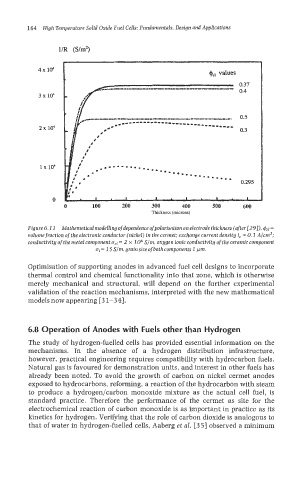
4x 104 +e, values
03
.*.----.*......
...*
-0-
---.*.*.
0.295
0 100 200 300 400 500 600
Thickness (microns)
Figure 6.11 Mathematical modelling of dependence ofpolarisation on electrode thickness (after 1291). =
volume fraction of the electronic conductor (nickel) in the cermet; exchange current density io = 0.1 A/cmZ;
conductivitg of the metal component a,l= 2 x lo6 S/m, oxygen ionic conductivity of the ceramic component
a, = I5 S/m, grain size of both components 1 pm.
Optimisation of supporting anodes in advanced fuel cell designs to incorporate
thermal control and chemical functionality into that zone, which is otherwise
merely mechanical and structural, will depend on the further experimental
validation of the reaction mechanisms, interpreted with the new mathematical
models now appearing [3 1-34].
6.8 Operation of Anodes with Fuels other than Hydrogen
The study of hydrogen-fuelled cells has provided essential information on the
mechanisms. In the absence of a hydrogen distribution infrastructure,
however, practical engineering requires compatibility with hydrocarbon fuels.
Natural gas is favoured for demonstration units, and interest in other fuels has
already been noted. To avoid the growth of carbon on nickel cermet anodes
exposed to hydrocarbons, reforming, a reaction of the hydrocarbon with steam
to produce a hydrogen/carbon monoxide mixture as the actual cell fuel, is
standard practice. Therefore the performance of the cermet as site for the
electrochemical reaction of carbon monoxide is as important in practice as its
kinetics for hydrogen. Verifying that the role of carbon dioxide is analogous to
that of water in hydrogen-fuelled cells, Aaberg et al. [3 51 observed a minimum