Page 185 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 185
162 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
0.40
a 0.20
UI
s4
2 0.00
a
c1
I
-0.20
-0.40
2.40 2.60 2.80 3.00 3.20
Real Part, Ohm
2.00
fl 1.00
U-
k
2 0.00
a
w
I
- 1 .oo
-2.00 1
2.00 3.00 4.00 5.00 6.00
Real Part, Ohm
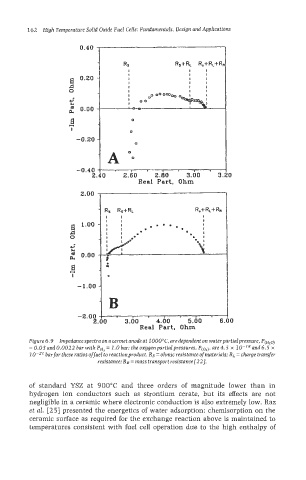
Figure 6.9 Impedance spectra on a cermet anode at 1 OOO"C, are dependent on waterpartiaIpressure. P(H20)
= 0.03 and 0.0022 bar with PH, = 1.0 bar: the oxygenpartialpressures, P(o,), are 4.5 x and 6.5 x
bar for these ratios offuel to reactionproduct. Rs = ohmic resistance of materials; RL = charge transfer
resistance; RR = mass transport resistance 1221.
of standard YSZ at 900°C and three orders of magnitude lower than in
hydrogen ion conductors such as strontium cerate, but its effects are not
negligible in a ceramic where electronic conduction is also extremely low. Raz
et al. [25] presented the energetics of water adsorption: chemisorption on the
ceramic surface as required for the exchange reaction above is maintained to
temperatures consistent with fueI cell operation due to the high enthalpy of