Page 186 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 186
Anodes 163
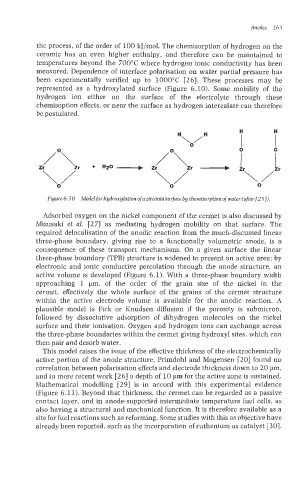
the process, of the order of 100 kJ/mol. The chemisorption of hydrogen on the
ceramic has an even higher enthalpy, and therefore can be maintained to
temperatures beyond the 700°C where hydrogen ionic conductivity has been
measured. Dependence of interface polarisation on water partial pressure has
been experimentally verified up to 1000°C [26]. These processes may be
represented as a hydroxylated surface (Figure 6.10). Some mobility of the
hydrogen ion either on the surface of the electrolyte through these
chemisoption effects, or near the surface as hydrogen intercalate can therefore
be postulated.
Figure 6.10 ikloclelfor hydroxylation of azirconiasurface by chemisorptionof water (after[25]).
Adsorbed oxygen on the nickel component of the cermet is also discussed by
Mizusaki et al. [27] as mediating hydrogen mobility on that surface. The
required delocalisation of the anodic reaction from the much-discussed linear
three-phase boundary, giving rise to a functionally volumetric anode, is a
consequence of these transport mechanisms. On a given surface the linear
three-phase boundary (TPB) structure is widened to present an active area: by
electronic and ionic conductive percolation through the anode structure, an
active volume is developed (Figure 6.1). With a three-phase boundary width
approaching 1 pm, of the order of the grain size of the nickel in the
cermet, effectively the whole surface of the grains of the cermet structure
within the active electrode volume is available for the anodic reaction. A
plausible model is Fick or Knudsen diffusion if the porosity is submicron,
followed by dissociative adsorption of dihydrogen molecules on the nickel
surface and their ionisation. Oxygen and hydrogen ions can exchange across
the three-phase boundaries within the cermet giving hydroxy1 sites, which can
then pair and desorb water.
This model raises the issue of the effective thickness of the electrochemicaIly
active portion of the anode structure. Primdahl and Mogensen [20] found no
correlation between polarisation effects and electrode thickness down to 20 pm,
and in more recent work [26] a depth of 10 pm for the active zone is sustained.
Mathematical modelling [29] is in accord with this experimental evidence
(Figure 6.11). Beyond that thickness, the cermet can be regarded as a passive
contact layer, and in anode-supported intermediate temperature fuel cells, as
also having a structural and mechanical function. It is therefore available as a
site for fuel reactions such as reforming. Some studies with this as objective have
already been reported, such as the incorporation of ruthenium as catalyst [30].