Page 200 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 200
Interconnects 177
conductivity and can survive such reducing conditions. Thus, the chromites are
quite unique and are the only oxides available for use as interconnects. The
LaCr03 doped with either Ca or Sr has sufficient conductivity in fuel atmospheres
to exceed 1 S/cm and therefore is preferred to Mg-doped LaCr03.
There has been some concern about the oxygen ion conductivity in
(La,SrCa)Cr03, particularly under reducing conditions, but studies by
Yokokawa et al. [9] and Singhal [lo] suggest that this is not a serious
problem since at 1000°C the oxygen diffusion coefficient appears to be less than
cm2/s. This would yield an ionic transport number of less than 0.01 even at
the most reducing conditions. Thus, oxygen permeation through the
interconnect should be minimal.
7.2.2 Thermal Expansion
It is important that the thermal expansion coefficients of all SOFC components
match well. This is particularly true for the dense components, the electrolyte
(most commonly yttria-stabilised zirconia, YSZ) and the interconnect. Table 7.3
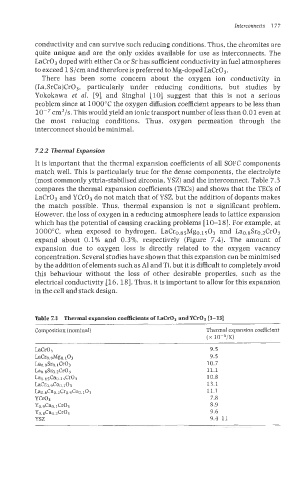
compares the thermal expansion coefficients (TECs) and shows that the TECs of
LaCr03 and YCr03 do not match that of YSZ, but the addition of dopants makes
the match possible. Thus, thermal expansion is not a significant problem.
However, the loss of oxygen in a reducing atmosphere leads to lattice expansion
which has the potential of causing cracking problems [lo-181. For example, at
1000°C, when exposed to hydrogen, LaCro.8&go.l 503 and Lao.8Sro.2Cr03
expand about 0.1% and 0.3%, respectively (Figure 7.4). The amount of
expansion due to oxygen loss is directly related to the oxygen vacancy
concentration. Several studies have shown that this expansion can be minimised
by the addition of elements such as A1 and Ti, but it is difficult to completely avoid
this behaviour without the loss of other desirable properties, such as the
electrical conductivity [16, 181. Thus, it is important to allow for this expansion
in the cell and stack design.
Table 7.3 Thermal expansion coefficients of LaCrO:, andYCr03 p-131
Composition (nominal) Thermal expansion coefficient
(x 10-6/K)
LaCr03 9.5
LaCro.gMg0.103 9.5
Lao.gSro.lCr03 10.7
Ladr0.2CrO3 11.1
h~Cao.3 003 10.8
La%.&o0.103 13.1
La0.8Ca0.2Cro.sCoo.lO3 11.1
YCr03 7.8
Yo.YC~O.IC~O~ 8.9
Yo.sC~O,~C~O~ 9.6
YSZ 9.4-11