Page 271 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 271
248 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
- - - - - screenprinted LSM-cathode on
0.0 0.2 0.4 0.6 0.8 1 .o 1.2
power density I (Wlcmq
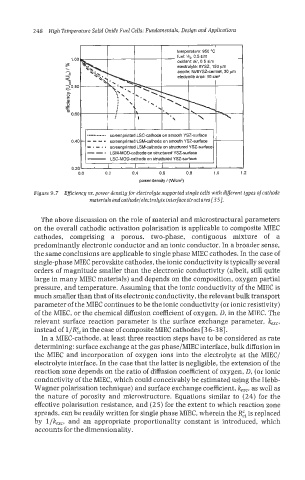
Figure 9.7 Efficiency vs. power densitg for electrolyte supported single cells with diferent types of cathode
materinls and cathodelelectrolgte interface structures [35].
The above discussion on the role of material and microstructural parameters
on the overall cathodic activation polarisation is applicable to composite MIEC
cathodes, comprising a porous, two-phase, contiguous mixture of a
predominantly electronic conductor and an ionic conductor. In a broader sense,
the same conclusions are applicable to single phase MIEC cathodes. In the case of
single-phase MIEC perovskite cathodes, the ionic conductivity is typically several
orders of magnitude smaller than the electronic conductivity (albeit, still quite
large in many MIEC materials) and depends on the composition, oxygen partial
pressure, and temperature. Assuming that the ionic conductivity of the MIEC is
much smaller than that of its electronic conductivity, the relevant bulk transport
parameter of the MIEC continues to be the ionic conductivity (or ionic resistivity)
of the MIEC, or the chemical diffusion coefficient of oxygen, D, in the MIEC. The
relevant surface reaction parameter is the surface exchange parameter, kesc,
instead of l/R:t in the case of composite MIEC cathodes [3 6-38].
In a MIEC-cathode, at least three reaction steps have to be considered as rate
determining: surface exchange at the gas phase/MIEC interface, bulk diffusion in
the MIEC and incorporation of oxygen ions into the electrolyte at the MIEC/
electrolyte interface. In the case that the latter is negligible, the extension of the
reaction zone depends on the ratio of diffusion coefficient of oxygen, D, (or ionic
conductivity of the MIEC, which could conceivably be estimated using the Hebb-
Wagner polarisation technique) and surface exchange coefficient, kcxc, as well as
the nature of porosity and microstructure. Equations similar to (24) for the
effective polarisation resistance, and (2 5) for the extent to which reaction zone
spreads, can be readily written for single phase MIEC, wherein the REt is replaced
by l/keXc, and an appropriate proportionality constant is introduced, which
accounts for the dimensionality.