Page 273 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 273
2 50 High Tempprature Solid Oxide Fuel Cplls: Fundamentals, Design and Applications
found in Chapter 6. The anodic exchange current density, similar to the cathodic
exchange current density, depends upon a number of parameters:
i: = f(TPB, partial pressure of hydrogen in the atmosphere,
oxygen vacancy concentration in the electrolyte, (27)
oxygen vacancy mobility, and temperature)
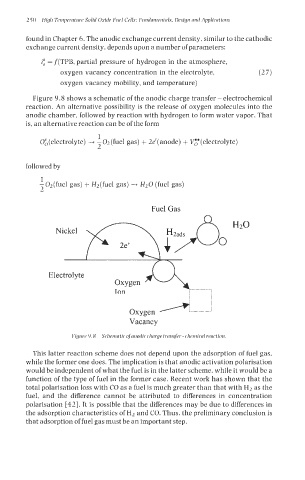
Figure 9.8 shows a schematic of the anodic charge transfer - electrochemical
reaction. An alternative possibility is the release of oxygen molecules into the
anodic chamber, followed by reaction with hydrogen to form water vapor. That
is, an alternative reaction can be of the form
1
Oz(electro1yte) -+ - 02 (fuel gas) + 2e’(anode) + Vy(electro1yte)
2
followed by
Fuel Gas
Nickel \
i
Oxygen /
..................
Vacancy
Figure 9.8 Schematic ofanodic charge transfer - chemical reaction.
This latter reaction scheme does not depend upon the adsorption of fuel gas,
while the former one does. The implication is that anodic activation polarisation
would be independent of what the fuel is in the latter scheme, while it would be a
function of the type of fuel in the former case. Recent work has shown that the
total polarisation loss with CO as a fuel is much greater than that with H2 as the
fuel, and the difference cannot be attributed to differences in concentration
polarisation [42]. It is possible that the differences may be due to differences in
the adsorption characteristics of H2 and CO. Thus, the preliminary conclusion is
that adsorption of fuel gas must be an important step.