Page 285 - Hydrocarbon Exploration and Production Second Edition
P. 285
272 Oil and Gas Processing
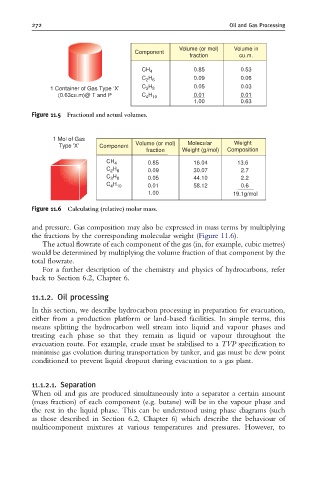
Volume (or mol) Volume in
Component
fraction cu.m.
CH 4 0.85 0.53
C H 0.09 0.06
2 6
1 Container of Gas Type ‘X’ C H 0.05 0.03
3 8
(0.63cu.m)@ T and P C H 0.01 0.01
4 10
1.00 0.63
Figure 11.5 Fractional and actual volumes.
1 Mol of Gas
Type 'X' Component Volume (or mol) Molecular Weight
fraction Weight (g/mol) Composition
CH 4 0.85 16.04 13.6
C H 0.09 30.07 2.7
2 6
C H 0.05 44.10 2.2
3 8
C H 0.01 58.12 0.6
4 10
1.00 19.1g/mol
Figure 11.6 Calculating (relative) molar mass.
and pressure. Gas composition may also be expressed in mass terms by multiplying
the fractions by the corresponding molecular weight (Figure 11.6).
The actual flowrate of each component of the gas (in, for example, cubic metres)
would be determined by multiplying the volume fraction of that component by the
total flowrate.
For a further description of the chemistry and physics of hydrocarbons, refer
back to Section 6.2, Chapter 6.
11.1.2. Oil processing
In this section, we describe hydrocarbon processing in preparation for evacuation,
either from a production platform or land-based facilities. In simple terms, this
means splitting the hydrocarbon well stream into liquid and vapour phases and
treating each phase so that they remain as liquid or vapour throughout the
evacuation route. For example, crude must be stabilised to a TVP specification to
minimise gas evolution during transportation by tanker, and gas must be dew point
conditioned to prevent liquid dropout during evacuation to a gas plant.
11.1.2.1. Separation
When oil and gas are produced simultaneously into a separator a certain amount
(mass fraction) of each component (e.g. butane) will be in the vapour phase and
the rest in the liquid phase. This can be understood using phase diagrams (such
as those described in Section 6.2, Chapter 6) which describe the behaviour of
multicomponent mixtures at various temperatures and pressures. However, to