Page 148 - Hydrogeology Principles and Practice
P. 148
HYDC04 12/5/05 5:36 PM Page 131
Environmental isotope hydrogeology 131
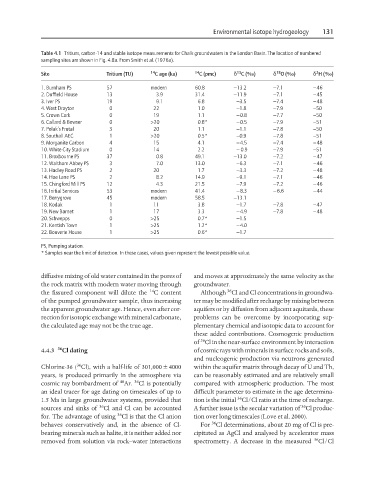
Table 4.1 Tritium, carbon-14 and stable isotope measurements for Chalk groundwaters in the London Basin. The location of numbered
sampling sites are shown in Fig. 4.8a. From Smith et al. (1976a).
2
18
13
Site Tritium (TU) 14 C age (ka) 14 C (pmc) d C (‰) d O (‰) d H (‰)
1. Burnham PS 57 modern 60.8 −13.2 −7.1 −46
2. Duffield House 13 3.9 31.4 −11.9 −7.1 −45
3. Iver PS 19 9.1 6.8 −3.5 −7.4 −48
4. West Drayton 0 22 1.0 −1.8 −7.9 −50
5. Crown Cork 0 19 1.1 −0.8 −7.7 −50
6. Callard & Bowser 0 >20 0.8* −0.5 −7.9 −51
7. Polak’s Frutal 3 20 1.1 −1.1 −7.8 −50
8. Southall AEC 1 >20 0.5* −0.9 −7.8 −51
9. Morganite Carbon 4 15 4.1 −4.5 −7.4 −48
10. White City Stadium 0 14 2.2 − 0.9 −7.9 −51
11. Broxbourne PS 37 0.8 49.1 −13.0 −7.2 −47
12. Waltham Abbey PS 3 7.0 13.0 −6.3 −7.1 −46
13. Hadley Road PS 2 20 1.7 −3.3 −7.2 −48
14. Hoe Lane PS 2 8.2 14.9 −9.1 −7.1 −46
15. Chingford Mill PS 12 4.3 21.5 −7.9 −7.2 −46
16. Initial Services 53 modern 41.4 −8.3 −6.6 −44
17. Berrygrove 45 modern 58.5 −13.1
18. Kodak 1 11 3.8 −1.7 −7.8 −47
19. New Barnet 1 17 3.3 −4.9 −7.8 −48
20. Schwepps 0 >25 0.7* −1.5
21. Kentish Town 1 >25 1.2* −4.0
22. Bouverie House 1 >25 0.6* −1.7
PS, Pumping station.
* Samples near the limit of detection. In these cases, values given represent the lowest possible value.
diffusive mixing of old water contained in the pores of and moves at approximately the same velocity as the
the rock matrix with modern water moving through groundwater.
14
36
the fissured component will dilute the C content Although Cl and Cl concentrations in groundwa-
of the pumped groundwater sample, thus increasing ter may be modified after recharge by mixing between
the apparent groundwater age. Hence, even after cor- aquifers or by diffusion from adjacent aquitards, these
rection for isotopic exchange with mineral carbonate, problems can be overcome by incorporating sup-
the calculated age may not be the true age. plementary chemical and isotopic data to account for
these added contributions. Cosmogenic production
36
of Cl in the near-surface environment by interaction
4.4.3 36 Cl dating of cosmic rays with minerals in surface rocks and soils,
and nucleogenic production via neutrons generated
36
Chlorine-36 ( Cl), with a half-life of 301,000 ± 4000 within the aquifer matrix through decay of U and Th,
years, is produced primarily in the atmosphere via can be reasonably estimated and are relatively small
36
40
cosmic ray bombardment of Ar. Cl is potentially compared with atmospheric production. The most
an ideal tracer for age dating on timescales of up to difficult parameter to estimate in the age determina-
36
1.5 Ma in large groundwater systems, provided that tion is the initial Cl/Cl ratio at the time of recharge.
36
36
sources and sinks of Cl and Cl can be accounted A further issue is the secular variation of Cl produc-
36
for. The advantage of using Cl is that the Cl anion tion over long timescales (Love et al. 2000).
36
behaves conservatively and, in the absence of Cl- For Cl determinations, about 20 mg of Cl is pre-
bearing minerals such as halite, it is neither added nor cipitated as AgCl and analysed by accelerator mass
36
removed from solution via rock–water interactions spectrometry. A decrease in the measured Cl/Cl