Page 144 - Hydrogeology Principles and Practice
P. 144
HYDC04 12/5/05 5:36 PM Page 127
Environmental isotope hydrogeology 127
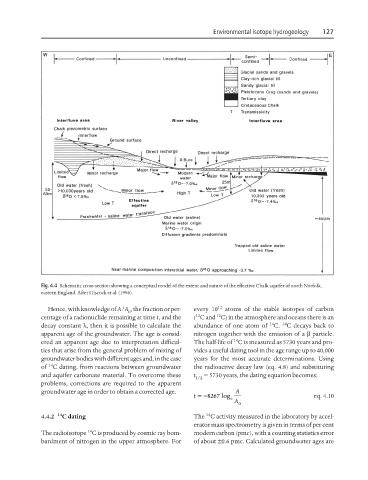
Fig. 4.4 Schematic cross-section showing a conceptual model of the extent and nature of the effective Chalk aquifer of north Norfolk,
eastern England. After Hiscock et al. (1996).
Hence, with knowledge of A/A , the fraction or per- every 10 12 atoms of the stable isotopes of carbon
0
12
13
centage of a radionuclide remaining at time t, and the ( C and C) in the atmosphere and oceans there is an
14
14
decay constant λ, then it is possible to calculate the abundance of one atom of C. C decays back to
apparent age of the groundwater. The age is consid- nitrogen together with the emission of a β particle.
14
ered an apparent age due to interpretation difficul- The half-life of C is measured as 5730 years and pro-
ties that arise from the general problem of mixing of vides a useful dating tool in the age range up to 40,000
groundwater bodies with different ages and, in the case years for the most accurate determinations. Using
14
of C dating, from reactions between groundwater the radioactive decay law (eq. 4.8) and substituting
and aquifer carbonate material. To overcome these t = 5730 years, the dating equation becomes:
1/2
problems, corrections are required to the apparent
groundwater age in order to obtain a corrected age. A
t =−8267 log eq. 4.10
e
A 0
14
4.4.2 14 C dating The C activity measured in the laboratory by accel-
erator mass spectrometry is given in terms of per cent
14
The radioisotope C is produced by cosmic ray bom- modern carbon (pmc), with a counting statistics error
bardment of nitrogen in the upper atmosphere. For of about ±0.6 pmc. Calculated groundwater ages are