Page 143 - Hydrogeology Principles and Practice
P. 143
HYDC04 12/5/05 5:36 PM Page 126
126 Chapter Four
inputs to groundwater, and also for separating stream
hydrographs into components of event (rainfall) and
pre-event (soil) water (see Section 5.7.1).
4.4 Age dating of groundwater
The age of a groundwater relates to the time when
an aquifer experiences recharge and is a measure of
the groundwater residence time. The exploitation
of groundwater resources at a rate in excess of the
time to replenish the aquifer storage will risk mining
the groundwater. Hence, knowledge of the age of
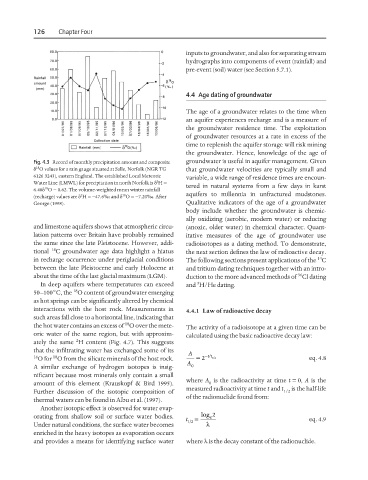
Fig. 4.3 Record of monthly precipitation amount and composite groundwater is useful in aquifer management. Given
18
δ O values for a rain gauge situated at Salle, Norfolk (NGR TG that groundwater velocities are typically small and
6126 3243), eastern England. The established Local Meteoric variable, a wide range of residence times are encoun-
Water Line (LMWL) for precipitation in north Norfolk is δ H =
2
18
6.48δ O − 0.62. The volume-weighted mean winter rainfall tered in natural systems from a few days in karst
2
18
(recharge) values are δ H =−47.6‰ and δ O =−7.20‰. After aquifers to millennia in unfractured mudstones.
George (1998). Qualitative indicators of the age of a groundwater
body include whether the groundwater is chemic-
ally oxidizing (aerobic, modern water) or reducing
and limestone aquifers shows that atmospheric circu- (anoxic, older water) in chemical character. Quant-
lation patterns over Britain have probably remained itative measures of the age of groundwater use
the same since the late Pleistocene. However, addi- radioisotopes as a dating method. To demonstrate,
14
tional C groundwater age data highlight a hiatus the next section defines the law of radioactive decay.
14
in recharge occurrence under periglacial conditions The following sections present applications of the C
between the late Pleistocene and early Holocene at and tritium dating techniques together with an intro-
about the time of the last glacial maximum (LGM). duction to the more advanced methods of Cl dating
36
In deep aquifers where temperatures can exceed and H/He dating.
3
18
50–100°C, the O content of groundwater emerging
as hot springs can be significantly altered by chemical
interactions with the host rock. Measurements in 4.4.1 Law of radioactive decay
such areas fall close to a horizontal line, indicating that
18
the hot water contains an excess of O over the mete- The activity of a radioisotope at a given time can be
oric water of the same region, but with approxim- calculated using the basic radioactive decay law:
2
ately the same H content (Fig. 4.7). This suggests
that the infiltrating water has exchanged some of its A
/
16 18 = − tt 12/
O for O from the silicate minerals of the host rock. 2 eq. 4.8
A similar exchange of hydrogen isotopes is insig- A 0
nificant because most minerals only contain a small
where A is the radioactivity at time t = 0, A is the
amount of this element (Krauskopf & Bird 1995). 0
measured radioactivity at time t and t is the half-life
Further discussion of the isotopic composition of 1/2
of the radionuclide found from:
thermal waters can be found in Albu et al. (1997).
Another isotopic effect is observed for water evap-
orating from shallow soil or surface water bodies. t = log e 2 eq. 4.9
/
Under natural conditions, the surface water becomes 12 λ
enriched in the heavy isotopes as evaporation occurs
and provides a means for identifying surface water where λ is the decay constant of the radionuclide.