Page 146 - Hydrogeology Principles and Practice
P. 146
HYDC04 12/5/05 5:36 PM Page 129
Environmental isotope hydrogeology 129
solution of calcite by weak carbonic acid (eq. 3.5) as
follows:
CaCO (lithic carbon) + H CO (biogenic carbon)
2
3
3
2+ −
→ Ca + 2HCO (sample carbon) eq. 4.11
3
If this reaction predominates, then the stoichiometry
14
of equation 4.11 predicts that the C activity of the
groundwater bicarbonate will be 50% of the modern
activity. In other words, because of dilution of the
sample with ‘dead’ carbon from soil and rock car-
bonate then, without correction, the apparent age of
the groundwater will appear older than it actually is.
To correct for this effect, one method is to use the
13
12
18
2
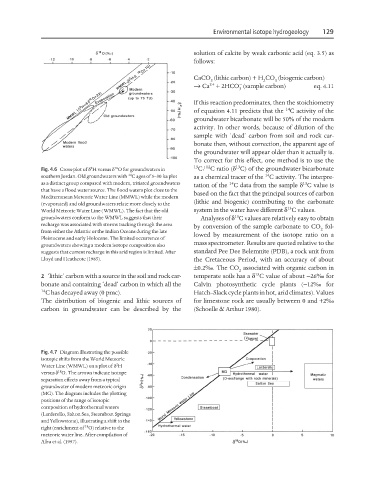
Fig. 4.6 Cross-plot of δ H versus δ O for groundwaters in 13 C/ C ratio (δ C) of the groundwater bicarbonate
14
14
southern Jordan. Old groundwaters with C ages of 5–30 ka plot as a chemical tracer of the C activity. The interpre-
as a distinct group compared with modern, tritiated groundwaters 14 13
tation of the C data from the sample δ C value is
that have a flood water source. The flood waters plot close to the
based on the fact that the principal sources of carbon
Mediterranean Meteoric Water Line (MMWL) while the modern
(lithic and biogenic) contributing to the carbonate
(evaporated) and old groundwaters relate more closely to the
13
World Meteoric Water Line (WMWL). The fact that the old system in the water have different δ C values.
13
groundwaters conform to the WMWL suggests that their Analyses of δ C values are relatively easy to obtain
recharge was associated with storms tracking through the area
by conversion of the sample carbonate to CO fol-
from either the Atlantic or the Indian Oceans during the late 2
lowed by measurement of the isotope ratio on a
Pleistocene and early Holocene. The limited occurrence of
mass spectrometer. Results are quoted relative to the
groundwaters showing a modern isotope composition also
suggests that current recharge in this arid region is limited. After standard Pee Dee Belemnite (PDB), a rock unit from
Lloyd and Heathcote (1985). the Cretaceous Period, with an accuracy of about
±0.2‰. The CO associated with organic carbon in
2
13
2 ‘lithic’ carbon with a source in the soil and rock car- temperate soils has a δ C value of about −26‰ for
bonate and containing ‘dead’ carbon in which all the Calvin photosynthetic cycle plants (−12‰ for
14
C has decayed away (0 pmc). Hatch–Slack cycle plants in hot, arid climates). Values
The distribution of biogenic and lithic sources of for limestone rock are usually between 0 and +2‰
carbon in groundwater can be described by the (Schoelle & Arthur 1980).
Fig. 4.7 Diagram illustrating the possible
isotopic shifts from the World Meteoric
2
Water Line (WMWL) on a plot of δ H
18
versus δ O. The arrows indicate isotope
separation effects away from a typical
groundwater of modern meteoric origin
(MG). The diagram includes the plotting
positions of the range of isotopic
composition of hydrothermal waters
(Larderello, Salton Sea, Steamboat Springs
and Yellowstone), illustrating a shift to the
18
right (enrichment of O) relative to the
meteoric water line. After compilation of
Albu et al. (1997).