Page 252 - Hydrogeology Principles and Practice
P. 252
HYDC06 12/5/05 5:34 PM Page 235
Groundwater quality and contaminant hydrogeology 235
observed in the transition from oxidizing conditions
in the background, uncontaminated groundwater to
the heavily polluted and highly reducing zone near
the lagoons at the base of the aquifer (Fig. 6.22b). These
zones follow the theoretical sequence of redox reac-
tions predicted from thermodynamic considerations
in a closed, organically polluted system (Table 3.11).
Heavy metals are attenuated near the lagoon as car-
bonates and as sulphides in the zone where sulphate
is reduced to sulphide.
Measurements of TOC suggested little gross
change in the organic carbon content of the pollution
plume at Villa Farm, although biotransformations
did occur. Aromatic hydrocarbons were broken down
with increasing distance from the lagoons as sug-
gested by the presence of benzoic acid derivatives
which were not present in the lagoons, but synthes-
ized from the primary disposal of phenol. The highly
reducing conditions throughout the plume below the
zone of sulphate reduction led to the production
of CH . However, at the same time, CH appeared to
4 4
be consumed during oxidation to CO in anaerobic
2
conditions in the overlying zone of sulphate reduction.
Where sulphate reduction was limited, such as at the
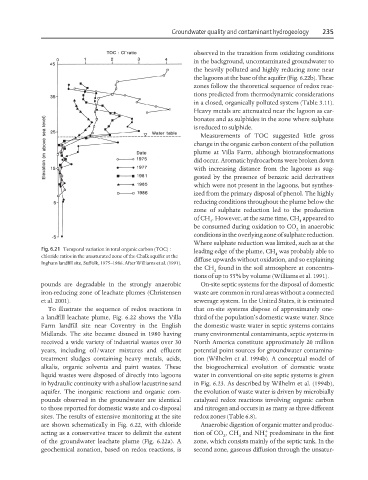
Fig. 6.21 Temporal variation in total organic carbon (TOC) :
leading edge of the plume, CH was probably able to
chloride ratios in the unsaturated zone of the Chalk aquifer at the 4
diffuse upwards without oxidation, and so explaining
Ingham landfill site, Suffolk, 1975–1986. After Williams et al. (1991).
the CH found in the soil atmosphere at concentra-
4
tions of up to 55% by volume (Williams et al. 1991).
pounds are degradable in the strongly anaerobic On-site septic systems for the disposal of domestic
iron-reducing zone of leachate plumes (Christensen waste are common in rural areas without a connected
et al. 2001). sewerage system. In the United States, it is estimated
To illustrate the sequence of redox reactions in that on-site systems dispose of approximately one-
a landfill leachate plume, Fig. 6.22 shows the Villa third of the population’s domestic waste water. Since
Farm landfill site near Coventry in the English the domestic waste water in septic systems contains
Midlands. The site became disused in 1980 having many environmental contaminants, septic systems in
received a wide variety of industrial wastes over 30 North America constitute approximately 20 million
years, including oil/water mixtures and effluent potential point sources for groundwater contamina-
treatment sludges containing heavy metals, acids, tion (Wilhelm et al. 1994b). A conceptual model of
alkalis, organic solvents and paint wastes. These the biogeochemical evolution of domestic waste
liquid wastes were disposed of directly into lagoons water in conventional on-site septic systems is given
in hydraulic continuity with a shallow lacustrine sand in Fig. 6.23. As described by Wilhelm et al. (1994b),
aquifer. The inorganic reactions and organic com- the evolution of waste water is driven by microbially
pounds observed in the groundwater are identical catalysed redox reactions involving organic carbon
to those reported for domestic waste and co-disposal and nitrogen and occurs in as many as three different
sites. The results of extensive monitoring at the site redox zones (Table 6.8).
are shown schematically in Fig. 6.22, with chloride Anaerobic digestion of organic matter and produc-
+
acting as a conservative tracer to delimit the extent tion of CO , CH and NH predominate in the first
2 4 4
of the groundwater leachate plume (Fig. 6.22a). A zone, which consists mainly of the septic tank. In the
geochemical zonation, based on redox reactions, is second zone, gaseous diffusion through the unsatur-