Page 255 - Hydrogeology Principles and Practice
P. 255
HYDC06 12/5/05 5:34 PM Page 238
238 Chapter Six
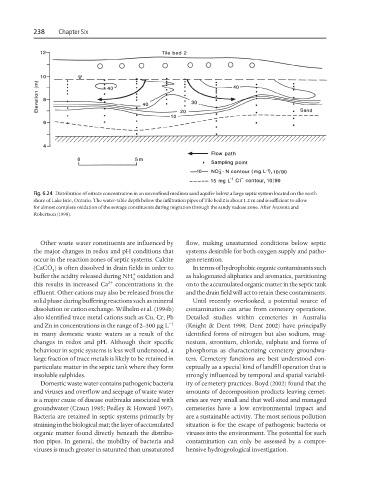
Fig. 6.24 Distribution of nitrate concentration in an unconfined medium sand aquifer below a large septic system located on the north
shore of Lake Erie, Ontario. The water-table depth below the infiltration pipes of Tile bed 2 is about 1.2 m and is sufficient to allow
for almost complete oxidation of the sewage constituents during migration through the sandy vadose zone. After Aravena and
Robertson (1998).
Other waste water constituents are influenced by flow, making unsaturated conditions below septic
the major changes in redox and pH conditions that systems desirable for both oxygen supply and patho-
occur in the reaction zones of septic systems. Calcite gen retention.
(CaCO ) is often dissolved in drain fields in order to In terms of hydrophobic organic contaminants such
3
+
buffer the acidity released during NH oxidation and as halogenated aliphatics and aromatics, partitioning
4
2+
this results in increased Ca concentrations in the on to the accumulated organic matter in the septic tank
effluent. Other cations may also be released from the and the drain field will act to retain these contaminants.
solid phase during buffering reactions such as mineral Until recently overlooked, a potential source of
dissolution or cation exchange. Wilhelm et al. (1994b) contamination can arise from cemetery operations.
also identified trace metal cations such as Cu, Cr, Pb Detailed studies within cemeteries in Australia
and Zn in concentrations in the range of 2–300 µgL −1 (Knight & Dent 1998; Dent 2002) have principally
in many domestic waste waters as a result of the identified forms of nitrogen but also sodium, mag-
changes in redox and pH. Although their specific nesium, strontium, chloride, sulphate and forms of
behaviour in septic systems is less well understood, a phosphorus as characterizing cemetery groundwa-
large fraction of trace metals is likely to be retained in ters. Cemetery functions are best understood con-
particulate matter in the septic tank where they form ceptually as a special kind of landfill operation that is
insoluble sulphides. strongly influenced by temporal and spatial variabil-
Domestic waste water contains pathogenic bacteria ity of cemetery practices. Boyd (2002) found that the
and viruses and overflow and seepage of waste water amounts of decomposition products leaving cemet-
is a major cause of disease outbreaks associated with eries are very small and that well-sited and managed
groundwater (Craun 1985; Pedley & Howard 1997). cemeteries have a low environmental impact and
Bacteria are retained in septic systems primarily by are a sustainable activity. The most serious pollution
straining in the biological mat; the layer of accumulated situation is for the escape of pathogenic bacteria or
organic matter found directly beneath the distribu- viruses into the environment. The potential for such
tion pipes. In general, the mobility of bacteria and contamination can only be assessed by a compre-
viruses is much greater in saturated than unsaturated hensive hydrogeological investigation.