Page 339 - Hydrogeology Principles and Practice
P. 339
HYDD01 12/5/05 5:31 PM Page 322
Composition of
Appendix 5
seawater and rainwater
A5.1 Seawater composition water and groundwater, and various processes of
removal. Important removal processes include losses
Ocean water consists of a complex solution with a to accumulating sediments by precipitation, sorption
total salt content, or salinity, of 3.5% (usually stated and organic activity, and reactions with basalt at
as 35‰ or 35 parts per thousand). The composi- mid-ocean ridges. Of the dissolved materials listed
tion of seawater is mainly controlled by a balance in Table A5.1, the most abundant and most constant
between the addition of dissolved material from river in concentration in all parts of the ocean are the
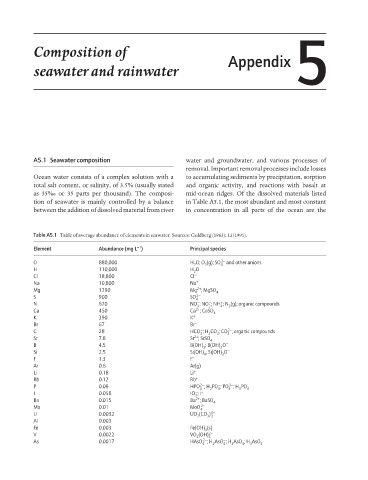
Table A5.1 Table of average abundance of elements in seawater. Sources: Goldberg (1963); Li (1991).
− −1
Element Abundance (mg L ) Principal species
O 880,000 H O; O (g); SO 2− and other anions
4
2
2
H 110,000 H O
2
Cl 18,800 Cl −
Na 10,800 Na +
2+
Mg 1290 Mg ; MgSO 4
S 900 SO 4 2−
−
−
+
N 670 NO ; NO ; NH ; N (g); organic compounds
4
2
2
3
2+
Ca 450 Ca ; CaSO 4
K 390 K +
Br 67 Br −
−
2−
C 28 HCO ; H CO ; CO ; organic compounds
3
3
2
3
2+
Sr 7.8 Sr ; SrSO 4
B 4.5 B(OH) ; B(OH) O −
2
3
Si 2.5 Si(OH) ; Si(OH) O −
4
3
F 1.3 F −
Ar 0.6 Ar(g)
Li 0.18 Li +
Rb 0.12 Rb +
−
2−
3−
P 0.09 HPO ; H PO ; PO ; H PO 4
3
4
4
4
2
− −
I 0.058 IO ; I
3
2+
Ba 0.015 Ba ; BaSO 4
Mo 0.01 MoO 4 2−
U 0.0032 UO (CO ) 4−
3 3
2
Al 0.003
Fe 0.003 Fe(OH) (s)
3
V 0.0022 VO (OH) 3 2−
2
2−
−
As 0.0017 HAsO ; H AsO ; H AsO ; H AsO 3
2
4
3
4
4
3